Translate this page into:
Practical immunohistochemistry of epithelial skin tumor
Correspondence Address:
Ahmed Alhumaidi
Department of Pathology (32), College of Medicine, King Saud University P.O. Box 2925, Riyadh 11461
Saudi Arabia
| How to cite this article: Alhumaidi A. Practical immunohistochemistry of epithelial skin tumor. Indian J Dermatol Venereol Leprol 2012;78:698-708 |
Abstract
Skin tumors are tumors arising from keratinocyte and from adnexal structures. Immunohistochemistry is very helpful in diagnosis of difficult cases in epithelial skin neoplasms, especially basal cell carcinoma (BCC) which is positive for BerEP4, a keratin marker, and mostly negative for epithelial membrane antigen (EMA). Squamous cell carcinoma cells are positive for EMA and cytokeratin, which are of higher molecular weight than those found in BCC. In contrast to BCC, trichoblastoma and trichoepithelioma are negative for androgen receptors. Of the malignant dermal spindle cell lesions, spindle cell squamous carcinoma is positive to 34 betaE12, desmoplasmic melanoma is positive to S100, and leiomyosarcoma is positive to desmin. Of the malignant pagetoid cells, Paget's disease is positive to CK7 and cam5.2, whereas the pagetoid variant of Bowen's disease is positive to CK 5/6. Melanoma in-situ is positive to both S100 and melan-A. Immunohistochemistry is an extremely valuable adjunct to standard morphologic diagnosis in diagnostic pathology. Diagnosis of epithelial tumor depends largely on morphological features but, in rare cases, immunohistochemical stains are needed for definitive diagnosis.Introduction
Skin tumors are tumors arising from keratinocyte and from adnexal structures. The latter either originate from hair follicles, eccrine, apocrine, sebaceous glands, or can be mixed. The histological diagnosis of these tumors is usually easy and straightforward. However, some cases are difficult, and show overlap features with other tumors, especially basal cell carcinoma (BCC). In this article, immunohistochemistry that is helpful in diagnosis of difficult cases in epithelial skin neoplasms were reviewed.
Keratinocytic Tumors
Basal cell carcinoma
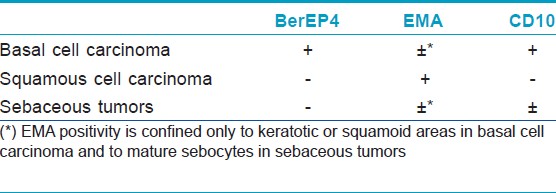
BCC is positive for BerEP4, a keratin marker [Figure - 1], and mostly negative for epithelial membrane antigen (EMA) [Table - 1]. In one study, all cases of BCC were positive for BerEP4, which differentiate it from squamous cell carcinomas (SCC). [1] Fan et al. reviewed the immunoreactivity of 51 cases of nodular BCCs and they found moderate or strong BerEP4 expression in all cases with never less than 20% of the tumor staining. Expression of EMA is uncommon in BCC (moderate or strong in 8% of tumors) and was confined to keratotic or squamoid areas. Immunohistochemistry of 25 sebaceomas revealed unequivocal negative expression of Ber-EP4 in 24 of 25 cases. A single case exhibited focal weak Ber-EP4 staining, predominantly in mature sebocytes and in < 10% of the tumor cells. EMA was not expressed in the germinative cells of sebaceoma, but was expressed strongly in approximately 50% of mature sebocytes in all cases and highlighted the cytoplasmic vacuoles. [2] Another study showed that Ber-EP4 reliably differentiates microcystic adnexal carcinoma (negative) from BCC (positive). [3] However, there is another study with contradictory data in which 10 cases of locally aggressive adnexal carcinoma showed Ber-EP4 positive membrane staining confined to the glandular areas. [4]
 |
| Figure 1: (a) Superficial basal cell carcinoma mimicking Bowen's disease (H and E, ×200), (b) Same tumor showing strong Ber-EP4 positivity (immunohistochemistry, ×100) |
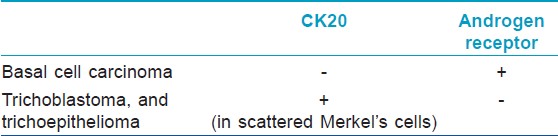
Cytokeratin 20 (CK20), a marker for Merkel cells, has been used to differentiate some forms of trichoblastoma, trichoepithelioma (TE), or fibroepitheliomas as these have scattered CK20-positive Merkel cells [Figure - 2] and [Figure - 3] compared to BCC where they are rare or absent. [5],[6] Izikson et al.′s study indicates that at least focal expression of androgen receptor (AR) was detected in 78% of BCCs and none of the trichoblastic tumors showed any AR immunoreactivity. [7] Immunohistochemical stains for AR and CK20 are useful to differentiate desmoplastic trichoepithelioma (DTE) from morpheaform/infiltrative BCC (mBCC). The AR-, CK20+ immunophenotype is sensitive (87%) and specific for DTE (100%). The AR+, CK20- immunophenotype is specific (100%) and moderately sensitive (61%) for mBCC [8] [Table - 2]. A recent study indicates that immunohistochemistry for ARs and CK20 are helpful, but interpretation is difficult in some DTEs when few cells are immunopositive for these markers. [9]
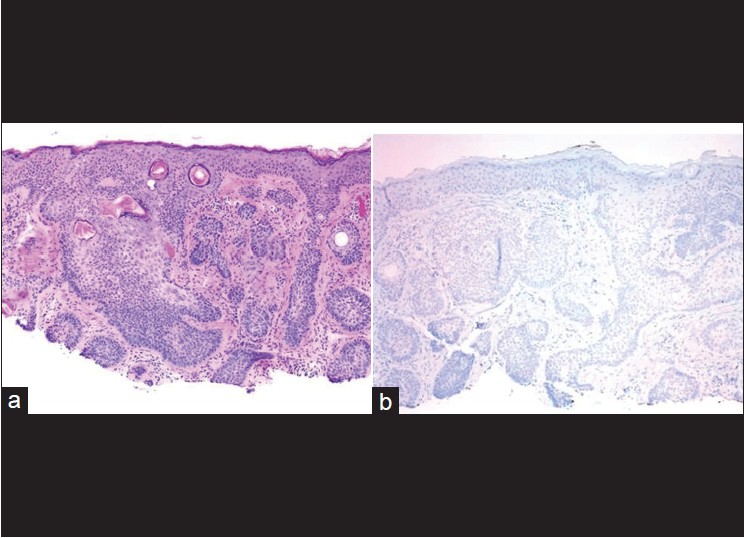 |
| Figure 2: (a) Basal cell carcinoma mimicking trichoepithelioma (H and E, ×100), (b) Cytokeratin 20 immunostain shows complete absence of Merkel cells (immunohistochemistry, ×200) |
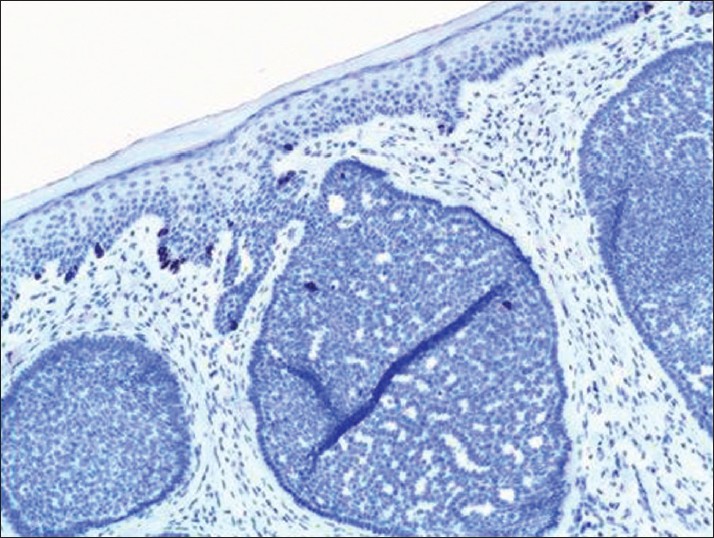 |
| Figure 3: As comparison, trichoblastoma demonstrates scattered cytokeratin 20-positive Merkel cells (immunohistochemistry, ×200) |
Wagoner et al.[10] studied CD10 expression of 14 and 13 cases of BCC and SCC, respectively. They found that CD10 was strongly expressed in 14 of 14 superficial BCCs and failed to express in 2 of 2 deeply infiltrative BCCs. CD10 was negative in the tumor cells in 13 of 13 superficially invasive SCCs and SCC in situ. CD10 expressed weakly in the surrounding stromal cells of 2 of 13 SCCs. [10]
A study of 32 patients with BCC showed that bcl-2 protein was positive in 26 patients (81.2%), bax protein was positive in 9 patients (28.1%), and p53 protein was positive in 13 patients (40.6%); there is a negative correlation between the expression of bcl-2 and bax protein (P<0.05). These findings suggest that apoptosis is suppressed which might cause the production of BCC. [11] An interesting aspect of BCC is that the forms that are more aggressive contain actin-positive cells, a finding that suggests that cell motility may play a role in tumor invasiveness. [12]
Squamous cell carcinoma
Variants of SCC, e.g., adenoid, mucin-producing acantholytic [Figure - 4], and spindle cell/desmoplastic [Figure - 5] SCC are sometimes difficult to diagnose by routine hematoxylin and eosin (HandE) stain. The best evidence of SCC is demonstration of intra- and/or extracellular keratinization. The cells in SCC are positive for EMA and cytokeratin. The keratins are of higher molecular weight than those found in BCC. [13]
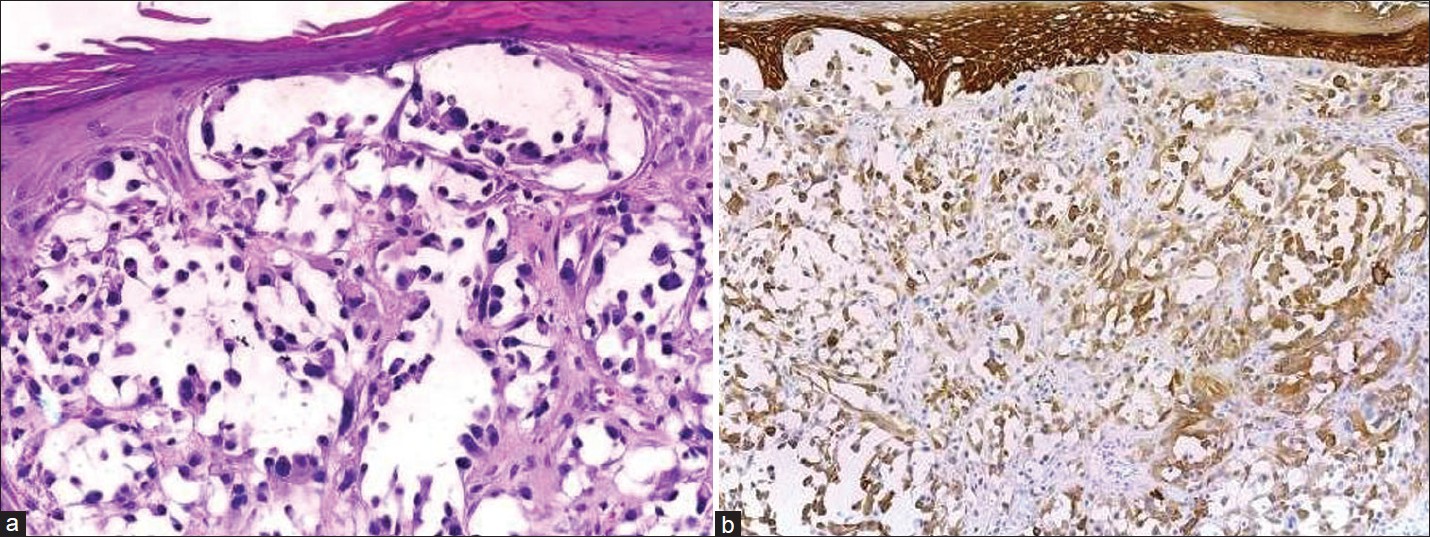 |
| Figure 4: (a) Acantholytic SCC forms spaces lined by atypical cells mimicking angiosarcoma ((H and E, ×400), (b) Acantholytic SCC shows high-molecular-weight cytokeratin positivity (immunohistochemistry, ×400) |
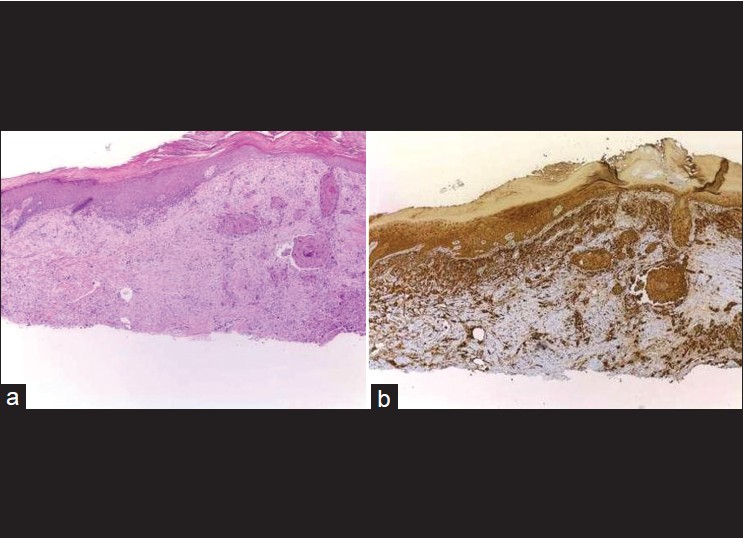 |
| Figure 5: (a) Desmoplastic SCC is sometimes difficult to distinguish from desmoplastic melanoma and atypical fibroxanthoma by routine (H and E, ×100), (b) Desmoplastic SCC shows positivity of high-molecular-weight cytokeratin in epidermis and neoplastic cells (immunohistochemistry, ×100) |
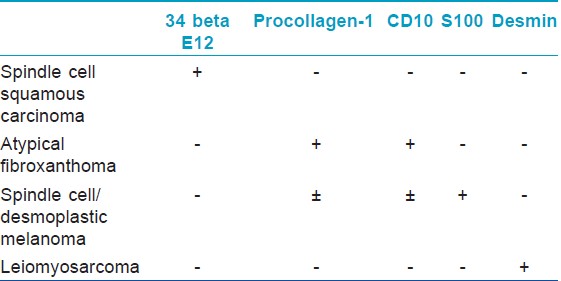
Cutaneous spindle cell squamous cell carcinoma (SCSCC) is an uncommon variant of SCC that must be distinguished from spindle cell/desmoplastic melanoma, cutaneous leiomyosarcoma, atypical fibroxanthoma (AFX), and scar. Morgan et al. studied 24 cases of SCC consisting of 12 spindle cell SCCs (SCSCCs), three AFXs, three leiomyosarcomas, three desmoplastic melanomas, and three scars were evaluated with a battery of immunohistochemical stains. The 12 SCSCCs show positive 34 beta E12 (12/12, 100%), p63 (10/12, 80%), AE1/AE3 (8/12, 67%), low-molecular-weight keratin (7/12, 58%), and P KER (4/12, 33%). The three AFXs were positive for CD68 and negative for all other stains, whereas the three leiomyosarcomas stained positively for desmin and smooth muscle actin and negatively for all other stains. The three melanomas stained positively for S-100 and negatively for all other immunohistochemistry. The scars were negative for all stains. So, this study shows that 34 beta E12 proved most promising in distinguishing SCSCC from the histological mimickers, AFX, spindle cell melanoma, scar, and leiomyosarcoma [14] [Table - 3]. CD10 and procollagen-1 immunostaining can be used as a useful adjunct to supplement the differentiation of AFX (positive) and spindle SCC (negative) within the context of an immunoperoxidase panel. [15]
Adenosquamous carcinoma is a rare variant of SCC arising from pluripotential cells related to acrosyringia, characterized by the formation of mucin-secreting glands. The tumors usually follow an aggressive course with the capacity for metastasis and local recurrence. The tumor cells are positive for cytokeratin and EMA, whereas those cells forming glands stain with carcinoembryonic antigen (CEA). [16]
Dotto and Glusac′s study showed that p63 was expressed diffusely in the nuclei of 100% (13/13) of SCSCC. Of controls, p63 showed focal labeling of two cutaneous leiomyosarcoma and two AFX. They concluded that p63 appears relatively specific to SCSCC and adds a useful nuclear marker to the available repertoire. [17]
CD44v6 and matrix metalloproteinase 1 (MMP-1), expressed in tumor cells, stromal cells respectively, are significant markers associated with the invasiveness of tumors in SCC and BCC of the skin, and that it will be helpful to evaluate the invasiveness by measuring the expression of these markers. [18]
Bowen disease
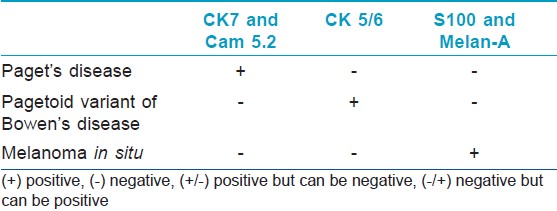
Bowen disease (BD) is a form of SCC in situ. It is a distinct clinicopathologic entity of the skin and mucocutaneous junction. The pagetoid variant of BD is sometimes difficult to distinguish from extramammary Paget disease. In the latter, mucicarmine, Cam 5.2, and CEA-positive tumor cells are present in the epidermis [16] [Table - 4].
BD shows increased nuclear expression of p27 (a protein associated with cellular quiescence) and Ki-67 (a marker of proliferation) [Figure - 6]. In one study, nuclear expression of p27 and Ki-67 was evaluated immunohistochemically in actinic keratosis (AK), BD, and SCC. They found that p27 labeling index was decreased in invasive aggregates of SCC (76.9± 1.1%) when compared with those of normal epidermis (97.2± 2.4%), AK (95.3 ± 1.4%), and BD (98.0± 0.5%). Ki-67 was expressed in a scattered to confluent linear pattern in the basal/parabasal cell layer of normal epidermis and AK. Keratinocytes in SCC exhibited Ki-67 in the peripheral layers of the neoplasm and frequently within the tumor aggregates. Ki-67 was observed in nuclei throughout the full thickness of the epidermis in BD. The staining pattern of Ki-67 in BD separated this entity from others under study. The combination pattern of p27 and Ki-67 staining can be used to support differentiation of AK, BD, and SCC. [19]
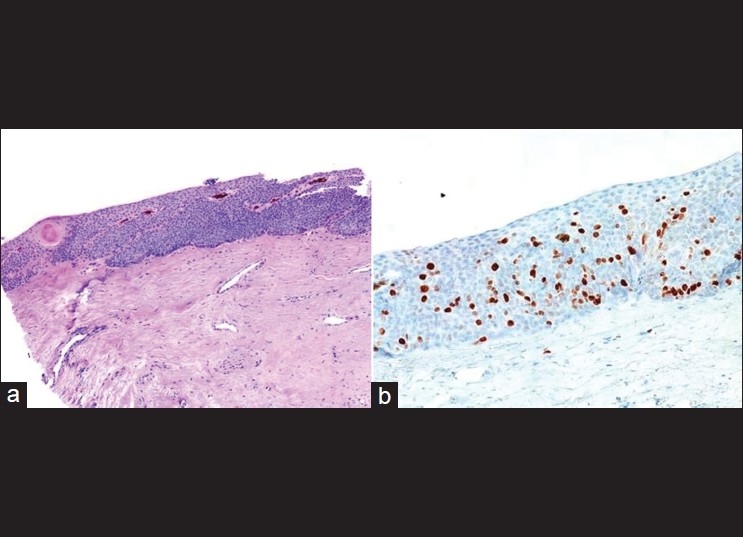 |
| Figure 6: (a) Bowen's disease of the nail (H and E, ×100), (b) Ki-67 immunostain shows high proliferation rate (Immunohistochemistry, ×100) |
BD features atypical squamous cells in all portions of the epidermis but initially leaves basal cells in palisades along the basement membrane. Saglam et al. have evaluated Ki-67 and P53 immunohistochemistry in AK and palisading basal cells (PBC) in BD. AK stained for Ki-67 and p53 antibodies only in lower portion of epidermis and included the basal layer. BD with typical PBCs stained positive for both markers throughout the epidermis, except for the basal layer [Figure - 7]. Four additional psoriatic biopsies stained positively for the two markers only in the basal and parabasal layers. Normal epidermis adjacent to the lesions in AK and BD biopsies stained sparsely in the basal layers. [20]
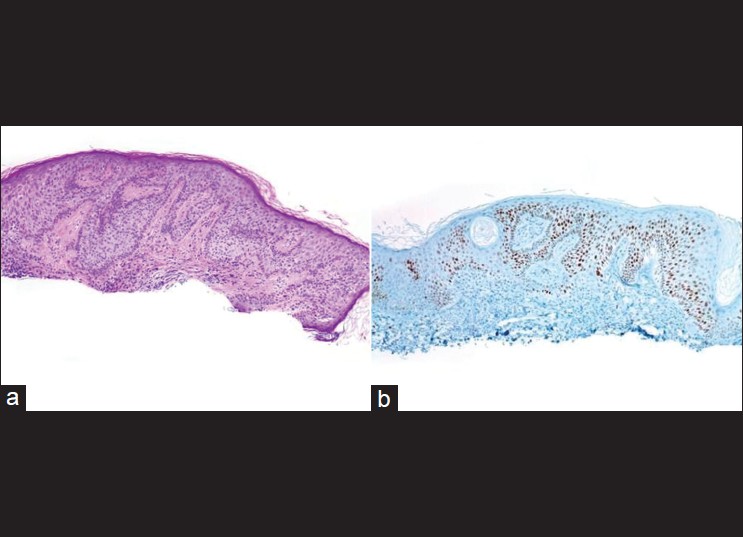 |
| Figure 7: (a) Bowen's disease mimicking seborrheic keratosis (H and E, ×100), (b) P53 immunostain shows a nearly full thickness epidermal positivity sparing the basal layer (immunohistochemistry, ×100) |
Actinic keratosis
AK is a common intraepidermal neoplasm of sun-damaged skin characterized by variable atypia of keratinocytes. Ber-EP4 show negative result in AK as opposed to superficial BCC. Tope et al.[21] showed that abnormal keratinocytes in all specimens of superficial BCC (5 of 5) were Ber-EP4 positive; and all AK (10 of 10) and squamous intraepithelial neoplasia (8 of 8) were Ber-EP4 negative. [21]
Keratoacanthoma
PCNA/MIB-1-labeled proliferating cells are found in the periphery of the squamous nests in keratoacanthoma (KA), in contrast to a more diffuse pattern in SCC. [15] In a study, 80% of the KAs showed nuclear staining with anti-p53 antibody, distributed along the outermost layers of the aggregates of neoplastic cells, while 60% of the SCCs were p53 positive. Eight percent of the regressing KA also showed p53 positivity. [22] The latter finding is used to differentiate regressing KA from normal and reactive epidermis.
A study showed that a high index of staining of p53 favors the diagnosis of subungual SCC over subungual keratoacanthoma (SUKA). SUKAs do not express Ki67 strongly, whereas some subungual SCCs do. They conclude that immunohistochemistry for p53 and Ki67 may help distinguish between a subungual SCC and a SUKA. [23]
Tumors with Differentiation toward Hair Follicle Structures
Trichoepithelioma and trichoblastoma
Trichoblastomas are benign neoplasms whose differentiation is toward the follicular germ-the embryonic structure that gives rise to the pilosebaceous-apocrine unit. TEs represent the most common form of trichoblastoma. Their most important differential diagnosis is with BCC, whose differentiation is similar. These tumors show scattered CK20-positive Merkel cells colonizing their peripheral layers [Figure - 3]. Merkel cells are sparsely scattered in the basal layer of the epidermis and in hair follicles, so that their presence in trichoblastomas is a sign of follicular differentiation. Benign trichoblastic neoplasms are negative for AR proteins, further aiding in their distinction from BCC, which often expressed them. In a study done by Katona et al., [24] immunohistochemistry for AR and CK20 was performed on 15 DTE and 31 mBCC. AR expression was seen in 13% (2/15) of DTE and 65% (20/31) of mBCC cases. CK20-positive Merkel cells were identified in 100% (15/15) of DTE and 3% (1/31) of mBCC. The expected pattern of AR-, CK20+ immunophenotype was present in 87% (13/15) of DTE cases. In mBCC, 61% (19/31) was AR+, CK20-. No DTE was AR+, CK20- and no mBCC was AR-, CK20+. [24] Another study concludes that immunohistochemistry for ARs and CK20 is helpful, but interpretation is difficult in some DTEs when few cells are immunopositive for these markers. This can be especially true in small biopsy specimens, which is a particular problem as these lesions are often present on the face.
CD10 staining pattern may be a useful adjunct marker in distinguishing between TE and BCC. In a study done by Pham et al., 12/13 cases (92%) of TE showed positive stromal immunoreactivity. Of these, eight cases also demonstrated positivity of the papilla, and two showed positivity of the basaloid cells. No TE demonstrated epithelial expression alone. On the other hand, expression of CD10 by basaloid cells was identified in 20/23 (87%) cases of BCC. Stromal positivity was also identified in three cases of BCC. [25]
Lum and Binder studied the proliferative rate of basal-cell carcinoma and TE in small biopsy specimens. They found that BCCs qualitatively showed a greater proliferative fraction (using the antibody, Ki-67) compared to TE (50.0 vs 13.0%), as well as over-expression of p53. BCCs marked by p21 demonstrated scattered nuclear positivity compared to the virtual absence of staining in the TE. [26] Bcl-2 stains BCC in a diffuse pattern, whereas all of the TEs in one study showed staining of the outermost epithelial layer. [27]
Pilomatricoma and pilomatrix carcinoma
These two neoplasms are proliferations of follicular matrical cells. The cells of the matrix rapidly proliferate and give rise to the inner root sheath and to the hair shaft. Pilomatricoma and pilomatrix carcinoma are positive for the beta-catenin immunostain. Hassanein and Glanz reviewed 21 cases of pilomatricoma and five cases of pilomatrix carcinoma. All 26 tumors displayed both nuclear and cytoplasmic staining of beta-catenin in the basaloid cells with focal membranous staining. Shadow cells were negative in all tumors. Normal control sections from the scalp displayed nuclear reactivity of the matrical cells, mostly concentrated in the supramatrical zone of hair follicles. [28]
Proliferating trichilemmal tumor
Proliferating trichilemmal tumor (PTT) shows p53 immunoreactivity that is not different from that seen in SCCs with trichilemmal differentiation (SCCT). On the other hand, p53 immunostaining is virtually absent in its much more common counterpart, trichilemmal cysts. The similar p53 immunoreactivity in both PTT and SCCT favors the interpretation of the former as carcinoma. [29] Most authors hold, however, that PTTs comprise a spectrum from neoplasms with little malignant potential to ones best considered as fully malignant carcinomas. [30]
Trichilemmoma and trichilemmal carcinoma
Trichilemmal carcinoma (TLC) is purportedly carcinomas that differentiate toward the outer root sheath, which has clear cells. Its distinction from clear cell SCC can be problematic, and only a few cases in which this diagnosis is considered are likely authentic. [31] In reports of TLC, the lesions express CK 1, 10, 14, and 17, suggesting that TLC differentiates toward follicular infundibulum. In a comparison of CK expression between TLC and trichilemmoma, the absence of CK 15 and 16 in TLC may be related to transformation from trichilemmoma to TLC. [32]
Desmoplastic trichilemmoma (DT) is a variant of trichilemmoma, characterized by a central prominent desmoplastic component, which may simulate invasive carcinoma if looked at out of context. In DT, epithelial tumor cells showed CD34 immunostaining in contrast to BCC and SCC. [33]
Tumors with Sweat Gland Differentiation
The sweat glands consist of ductal and secretory portions. Only the secretory portion of both eccrine and apocrine glands expresses cytokeratins of low molecular weight such as CK7 and CAM 5.2. EMA and CEA stain the luminal aspect of the duct and show focal and somewhat weaker staining of the luminal aspect of the secretory glands. The myoepithelial cells are positive for S100 and smooth muscle actin (SMA). S100 also stain some secretory cells of eccrine glands.
Normal apocrine glands are stained with lysozyme, CD-15 (Leu M1), and gross cystic disease fluid protein-15 (GCDFP-15), while eccrine glands were positive only for GCDFP-15. In neoplastic tissue thought to be from apocrine tumors, antibodies rose against lysozyme and GCDFP-15 has a greater specificity (100%) for apocrine differentiation, while Leu M1 has a greater sensitivity (88%). [34]
There are histologic and immunophenotypic similarities between sweat gland carcinoma and breast cancer. Swanson et al. [35] assessed estrogen receptor protein (ERP) immunoreactivity in sweat gland tumors. They found that ERP was detected in 10 of 33 eccrine carcinomas, 3 of 10 eccrine hidradenomas, each of two examples of hidradenoma papilliferum, and 2 of 3 chondroid syringomas. Among immunoreactive eccrine neoplasms, eight of 10 carcinomas occurred in males. Another study done by Offidani et al. revealed that almost all the anogenital sweat glands showed immunoreactivity for estrogen receptor and, more weakly, for progesterone receptor, with immunolabeled nuclear area ranging from 10% to 90%. Conversely, conventional sweat glands did not show any nuclear staining. [35] Coexpression of cytokeratin and vimentin may be frequently found in a variety of benign and malignant sweat gland tumors. In the majority of these neoplasms, vimentin-positive cells correspond to myoepithelial cells, as indicated by coexpression with alpha- SMA. [36]
In cylindroma, cytokeratin 7 predominantly labels the central basaloid cells, and SMA stain peripheral myoepithelial cells. Cylindromas do not express CK20, gross cystic disease fluid protein 15, or estrogen or progesterone receptor. [37],[38],[39] Recent study by Missall et al. concluded that both cylindromas and spiradenomas express CK7, a sensitive marker for the secretory coil [Figure - 8]. They also found that syringomas express both CK6 (a marker for the inner ductal cells) and CK10 (a marker for the middle ductal cells). None of the studied tumors expressed SMA or CD10 (markers for myoepithelial cells). [40]
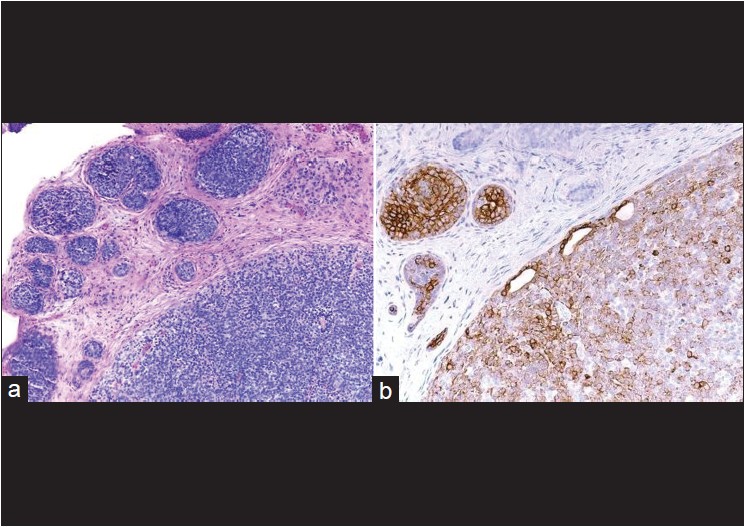 |
| Figure 8: (a) Spiradenoma, well-circumscribed aggregates of blue tumor cells are seen in the dermis (H and E, ×100), (b) Cytokeratin 7 positivity indicates sweat gland ductal origin (immunohistochemistry, ×100) |
The tubular cells in both spiradenomas and cylindromas express human milk fat globulin and lysozyme, two markers associated with apocrine differentiation, [41] S100 and CD1a show scattered langerhans cells within these lesions, a finding that is confirmed by the electron microscopic observation of Birbeck granules. [38],[42]
Poromas are benign adnexal neoplasms with terminal ductal differentiation. Although historically considered a neoplasm of eccrine differentiation, poromas can show either eccrine or apocrine lineage. [34] Most of the poroid cells are stained positively with antibodies against CKs 5 and 14, but none to very few cells (<5%) immunoreacted with antibodies against CKs 8/18 and CEA. Cuticular cells show a somewhat similar immunostaining pattern as poroid cells. The luminal edges of cuticle cells react with CEA. [43] Immunolabeling for EMA is present in the tumor cells of poroma. Thus, it is suggested that the constituent cells of these tumors originate from the outer cells of the intraepidermal and/or the upper portion of the intradermal eccrine ducts, as these cells are positive for EMA as well. There is little immunolabeling for EMA on the tumor cells of seborrheic keratosis and BCC. [44]
The tumor cells of mixed tumor or chondroid syringoma are positive for cytokeratins (AE1+AE3). Glandular cells lining tubules are CK7 positive. The myoepithelial cells are S-100 protein, neuron-specific enolase, and glial fibrillary acidic protein. [45]
Malignant sweat gland neoplasms
There are many recognized WHO types of sweat gland carcinomas. Superficial biopsy specimens of microcystic adnexal carcinoma can simulate infiltrative BCC and DTE. Hoang et al. found that the majority of microcystic adnexal carcinomas (MAC) (92%) and DTEs (100%) expressed CK15, whereas infiltrative BCCs and SCCs were all negative. [46] Another study showed that Ber-EP4 did not label any of the MAC (0/13), whereas 28/28 cases of BCCs were Ber-EP4 positive. 12/16 of DTEs were immunoreactive with Ber-EP4. [25] However, there is another study with contradictory data in which 10 cases of locally aggressive adnexal carcinoma showed Ber-EP4-positive membrane staining confined to the glandular areas. [26]
CEA and EMA highlight the ducts and the intracytoplasmic lumina of porocarcinoma and hidradenocarcinoma. [34],[47]
Primary mucinous carcinoma of the skin can be mistaken histologically for a metastasis from extracutaneous sites, particularly the breast, or the gastrointestinal tract. Primary mucinous carcinoma of the skin is positive for cytokeratin 7, S100 protein, estrogen, and progesterone receptors, while negative for CK20. [48] Primary cutaneous mucinous carcinomas often have an area of carcinoma in situ, in which there are actin-positive myoepithelial cells at the edges of some of the aggregates. [49]
The most reliable histopathologic criteria for identifying apocrine skin carcinoma are decapitation secretion, periodic acid-Schiff-positive diastase-resistant material in the cells or lumen, and immunoreactivity with gross cystic disease fluid protein 15. Apocrine carcinoma also expresses EMA and variably CEA. [50] Tubular carcinoma is the malignant counterpart of tubular adenoma, featuring apocrine differentiation with prominent tubular structures. Tubular carcinoma is positive for low-molecular-weight cytokeratins and the luminal cells express EMA and gross cystic disease fluid protein 15 (GCDFP-15). Expression of CEA is variable. [34]
Mammary and extramammary Paget disease
Immunohistochemistry has been useful not only in the diagnosis of Paget′s disease but also in attempting to clarify the cell of origin in Paget′s disease. The most useful keratin markers for primary mammary and extramammary Paget disease are CAM5.2 and CK7 because they stain >90% of Paget cells but do not react with epidermal or mucosal keratinocytes [51] [Table - 4]. CK7 also marks some Toker cells and Merkel cells, so morphological assessment of the labeled cells is necessary. [52] Secondary extramammary Paget disease arises from underlying adenocarcinomas, mostly from gastrointestinal tract. Such cases express CK20 and show negativity for CK7. GCDFP-15 is strongly expressed in cases of vulval and perianal extramammary Paget′s disease without an underlying internal malignancy, and much less frequently in cases with an associated malignancy. [53] Rare cases of extramammary Paget′s disease are associated with proastatic adenocarcinoma and are prostatic-specific antigen (PSA) positive, [53],[54] or from the urinary bladder. [55],[56]
Tumors with Sebaceous Differentiation
There is considerable overlap between the histological features of sebaceoma and BCC. Fan et al. showed that 24 of 25 sebaceomas are negative for Ber-EP4. EMA was not expressed in the germinative cells of sebaceoma, but was expressed strongly in approximately 50% of mature sebocytes in all cases and highlighted the cytoplasmic vacuoles. In contrast, all the [46] cases of BCC show moderate or strong BerEP4 expression with never less than 20% of the tumor staining. Expression of EMA was uncommon in BCC (moderate or strong in 8% of cases) and was confined to keratotic or squamoid areas. [57] Immunohistochemical staining for AR is found to be a reliable marker of sebaceous differentiation. Bayer-Garner et al. found that all sebaceous neoplasms show diffuse positive nuclear AR staining, whereas approximately 60% of BCCs showed only focal positivity for nuclear AR immunoreactivity. Clear cell acanthomas and SCCs were uniformly negative. [58]
Primary vs Metastatic Carcinoma of the Skin
Distinction of primary skin adnexal carcinomas from cutaneous metastasis of adenocarcinoma is sometimes challenging. In a study, podoplanin is positive in primary skin adnexal carcinomas and negative in metastatic adenocarcinomas. [59] Another study revealed that all primary cutaneous carcinomas, including adenocarcinomas, expressed p63. In contrast, none of the metastatic adenocarcinomas to the skin was positive for p63. [60] CK5/6 can also be used to distinguish primary skin adnexal carcinomas from metastatic adenocarcinoma. Plumb et al.′s study concluded that CK 5/6 was expressed by most (97%) of cutaneous adnexal neoplasms, while only 33% of metastatic adenocarcinomas showed positive expression. [61]
Estrogen and progesterone receptors and anti-gross cystic disease fluid protein 15 (BRST-2) are positive in metastatic breast carcinoma and can be positive in primary eccrine and apocrine neoplasms. Thus, these markers do not differentiate between primary and metastatic breast carcinoma. [62]
There are some more specific markers that help pinpoint the origin of metastases to the skin. Examples of these include thyroglobulin (thyroid carcinoma), TTF-1 (thyroid or lung carcinoma), renal cell carcinoma antigen (renal cell carcinoma), and glypican-3 for hepatocellular carcinoma [Table - 5].

Conclusion
Immunohistochemistry is an extremely valuable adjunct to standard morphologic diagnosis in diagnostic pathology. Diagnosis of epithelial tumor depends largely on morphological features. In rare cases, immunohistochemical stains are needed for definitive diagnosis. Sometimes, a panel of markers is recommended instead of a single immunostain. A careful interpretation of the result and correlation with histological features will guide to correct and specific diagnosis.
| 1. |
Tellechea O, Reis JP, Domingues JC, Baptista AP. Monoclonal antibody Ber EP4 distinguishes basal-cell carcinoma from squamous-cell carcinoma of the skin. Am J Dermatopathol 1993;15:452-55.
[Google Scholar]
|
| 2. |
Fan YS, Carr RA, Sanders DS, Smith AP, Lazar AJ, Calonje E. Characteristic Ber-EP4 and EMA expression in sebaceoma is immunohistochemically distinct from basal cell carcinoma. Histopathology 2007;51:80-6.
[Google Scholar]
|
| 3. |
Krahl D, Sellheyer K. Monoclonal antibody Ber-EP4 reliably discriminates between microcystic adnexal carcinoma and basal cell carcinoma. J Cutan Pathol 2007;34:782-7.
[Google Scholar]
|
| 4. |
Smith KJ, Williams J, Corbett D, Skelton H. Microcystic adnexal carcinoma: An immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol 2001;25:464- 71.
[Google Scholar]
|
| 5. |
Abesamis-Cubillan E, Shabrawi- Caelen L, LeBoit PE. Merked cells and sclerosing epithelial neoplasms. Am J Dermatopathol 2000;22: 311-5.
[Google Scholar]
|
| 6. |
Schulz T, Hartschuh W. Merkel cells are absent in basal cell carcinomas but frequently found in trichoblastomas. An immunohistochemical study. J Cutan Pathol 1997;24:14-24.
[Google Scholar]
|
| 7. |
Izikson L, Bhan A, Zembowicz A. Androgen receptor expression helps to differentiate basal cell carcinoma from benign trichoblastic tumors. Am J Dermatopathol 2005;27:91-5.
[Google Scholar]
|
| 8. |
Costache M, Bresch M, Böer A. Does the panel of cytokeratin 20 and androgen receptor antibodies differentiate desmoplastic trichoepithelioma from morpheaform/infiltrative basal cell carcinoma? J Cutan Pathol 2008;35:174-9.
[Google Scholar]
|
| 9. |
Costache M, Bresch M, Böer A. Desmoplastic trichoepithelioma versus morphoeic basal cell carcinoma: A critical reappraisal of histomorphological and immunohistochemical criteria for differentiation. Histopathology 2008;52:865-76.
[Google Scholar]
|
| 10. |
Wagoner J, Keehn C, Morgan MB CD-10 immunostaining differentiates superficial basal cell carcinoma from cutaneous squamous cell carcinoma. Am J Dermatopathol 2007;29:555- 8.
[Google Scholar]
|
| 11. |
Yan L, Chen M, Yu G. Expression of bcl-2, bax, and p53 protein in the basal cell carcinoma. Hunan Yi Ke Da Xue Xue Bao 1999;24:179-80.
[Google Scholar]
|
| 12. |
Christian MM, Moy RL, Wagner RF, Yen-Moore A. A correlation of alpha-smooth muscle actin and invasion in micronodular basal cell carcinoma. Dermatol Surg 2001;27:441-5.
[Google Scholar]
|
| 13. |
Nadji M. Immunoperoxidase techniques. II. Application to cutaneous neoplasms. Am J Dermatopathol 1986;8:124-9.
[Google Scholar]
|
| 14. |
Morgan MB, Purohit C, Anglin TR. Immunohistochemical distinction of cutaneous spindle cell carcinoma. Am J Dermatopathol 2008;30:228-32.
[Google Scholar]
|
| 15. |
De Feraudy S, Mar N, McCalmont TH. Evaluation of CD10 and procollagen one expression in atypical fibroxanthoma and dermatofibroma. Am J Surg Pathol 2008;32:1111-22.
[Google Scholar]
|
| 16. |
LeBoit PE, Burg G, Weedon D, Sarasain A, editors. World Health Organization Classification of Tumors. Pathology and Genetics of Skin Tumors. Lyon: IARC Press; 2006.
[Google Scholar]
|
| 17. |
Dotto JE, Glusac EJ. p63 is a useful marker for cutaneous spindle cell squamous cell carcinoma. J Cutan Pathol 2006;33:413-7.
[Google Scholar]
|
| 18. |
Son KD, Kim TJ, Lee YS, Park GS, Han KT, Lim JS, et al. Comparative analysis of immunohistochemical markers with invasiveness and histologic differentiation in squamous cell carcinoma and basal cell carcinoma of the skin. J Surg Oncol 2008;97:615-20.
[Google Scholar]
|
| 19. |
Oh CW, Penneys N. P27 and mib1 expression in actinic keratosis, Bowen disease, and squamous cell carcinoma. Am J Dermatopathol 2004;26:22-6.
[Google Scholar]
|
| 20. |
Saglam O, Salama M, Meier F, Chaffins M, Ma C, Ormsby A, et al. Immunohistochemical staining of palisading basal cells in Bowen's disease and basal involvement in actinic keratosis: Contrasting staining patterns suggest different cells of origin. Am J Dermatopathol 2008;30:123-6.
[Google Scholar]
|
| 21. |
Tope WD, Nowfar-Rad M, Kist DA. Ber-EP4-positive phenotype differentiates actinic keratosis from superficial basal cell carcinoma. Dermatol Surg 2000;26:415-8.
[Google Scholar]
|
| 22. |
Kerschmann RL, McCalmont TH, LeBoit PE. p53 oncoprotein expression and proliferation index in keratoacanthoma and squamous cell carcinoma. Arch Dermatol 1994;130:181-6.
[Google Scholar]
|
| 23. |
Connolly M, Narayan S, Oxley J, de Berker DA. Immunohistochemical staining for the differentiation of subungual keratoacanthoma from subungual squamous cell carcinoma. Clin Exp Dermatol 2008;33:625-8.
[Google Scholar]
|
| 24. |
Katona TM, Perkins SM, Billings SD. Does the panel of cytokeratin 20 and androgen receptor antibodies differentiate desmoplastic trichoepithelioma from morpheaform/infiltrative basal cell carcinoma? J Cutan Pathol 2008;35:174-9.
[Google Scholar]
|
| 25. |
Pham TT, Selim MA, Burchette JL Jr, Madden J, Turner J, Herman C. CD10 expression in trichoepithelioma and basal cell carcinoma. J Cutan Pathol 2006;33:123-8.
[Google Scholar]
|
| 26. |
Lum CA, Binder SW. Proliferative characterization of basal-cell carcinoma and trichoepithelioma in small biopsy specimens. J Cutan Pathol 2004;31:550-4.
[Google Scholar]
|
| 27. |
Poniecka AW, Alexis JB. An immunohistochemical study of basal cell carcinoma and trichoepithelioma. Am J Dermatopathol 1999;21:332-6.
[Google Scholar]
|
| 28. |
Hassanein AM, Glanz SM. Beta-catenin expression in benign and malignant pilomatrix neoplasms. Br J Dermatol 2004;150:511-6.
[Google Scholar]
|
| 29. |
Fernández-Figueras MT, Casalots A, Puig L, Llatjós R, Ferrándiz C, Ariza A. Proliferating trichilemmal tumour: p53 immunoreactivity in association with p27Kip1 over-expression indicates a low-grade carcinoma profile. Histopathology 2001;38:454-7.
[Google Scholar]
|
| 30. |
Ye J, Nappi O, Swanson PE, Patterson JW, Wick MR. Proliferating pilar tumors: A clinicopathologic study of 76 cases with a proposal for definition of benign and malignant variants. Am J Clin Pathol 2004;122:566-74.
[Google Scholar]
|
| 31. |
Dalton SR, LeBoit PE. Squamous cell carcinoma with clear cells: How often is there evidence of tricholemmal differentiation? Am J Dermatopathol 2008;30:333-9.
[Google Scholar]
|
| 32. |
Kurokawa I, Senba Y, Nishimura K, Habe K, Hakamada A, Isoda K, et al. Cytokeratin expression in trichilemmal carcinoma suggests differentiation towards follicular infundibulum. In Vivo 2006;20:583-5.
[Google Scholar]
|
| 33. |
Illueca C, Monteagudo C, Revert A, Llombart-Bosch A. Diagnostic value of CD34 immunostaining in desmoplastic trichilemmoma. J Cutan Pathol 1998;25:435-9.
[Google Scholar]
|
| 34. |
Ansai S, Koseki S, Hozumi Y, Kondo S. An immunohistochemical study of lysozyme, CD-15 (Leu M1), and gross cystic disease fluid protein-15 in various skin tumors. Assessment of the specificity and sensitivity of markers of apocrine differentiation. Am J Dermatopathol 1995;17:249-55.
[Google Scholar]
|
| 35. |
Swanson PE, Mazoujian G, Mills SE, Campbell RJ, Wick MR. Immunoreactivity for estrogen receptor protein in sweat gland tumors. Am J Surg Pathol 1991;15:835-41.
[Google Scholar]
|
| 36. |
Kato, Ueno H. Eccrine hidrocystoma: two cases of Robinson and Smith types. J Dermatol 1992;19:493-7.
[Google Scholar]
|
| 37. |
Demirkesen C, Hoede N, Moll R. Epithelial markers and differentiation in adnexal neoplasms of the skin: An immunohistochemical study including individual cytokeratins. J Cutan Pathol 1995;22:518-35.
[Google Scholar]
|
| 38. |
Albores-Saavedra J, Heard SC, McLaren B, Kamino H, Witkiewicz AK. Cylindroma (dermal analog tumor) of the breast: A comparison with cylindroma of the skin and adenoid cystic carcinoma of the breast. J Cutan Pathol 1995;22:518-35.
[Google Scholar]
|
| 39. |
Wiley EL, Milchgrub S, Freeman RG, Kim ES, sweat gland adenomas: Immunohistochemical study with emphasis on myoepithelial differentiation. J Cutan Pathol 1993;20:337-43.
[Google Scholar]
|
| 40. |
Missall TA, Burkemper NM, Jensen SL, Hurley MY. Immunohistochemical differentiation of four benign eccrine tumors. J Cutan Pathol 2009;36:190-6.
[Google Scholar]
|
| 41. |
Meybehm M, Fischer HP. Spiradenoma and dermal cylindroma: comparative immunohistochemical analysis and histogenetic considerations. Am J Dermatopathol 1997;19:154-61.
[Google Scholar]
|
| 42. |
Ohtsuki Y, Ohtsuka H, Kurabayashi A, Iguchi M, Matsumoto M, Takeuchi T, et al. Immunohistochemical and electron microscopic studies of Langerhans cells in a case of multiple eccrine spiradenomas. Med Mol Morphol 2007;40:221-5.
[Google Scholar]
|
| 43. |
Liu HN, Chang YT, Chen CC, Huang CH. Histopathological and immunohistochemical studies of poroid hidradenoma. Arch Dermatol Res 2006;297:319-23.
[Google Scholar]
|
| 44. |
Takanashi M, Urabe A, Nakayama J, Hori Y. Distribution of epithelial membrane antigen in eccrine poroma. Dermatologica 1991;183:187-90.
[Google Scholar]
|
| 45. |
Takahashi H, Ishiko A, Kobayashi M, Tanikawa A, Takasu H, Md MT. Malignant chondroid syringioma with bone invasion: A case report and review of the literature. Am J Dermatopathol 2004;26:403-6.
[Google Scholar]
|
| 46. |
Hoang MP, Dresser KA, Kapur P, High WA, Mahalingam M. Microcystic adnexal carcinoma: An immunohistochemical reappraisal. Mod Pathol 2008;21:178-85.
[Google Scholar]
|
| 47. |
Robson A, Greene J, Ansari N, Kim B, Seed PT, McKee PH, et al. Eccrine porocarcinoma (malignant eccrine poroma): A clinicopathologic study of 69 cases. Am J Surg Pathol 2001;25:710-20.
[Google Scholar]
|
| 48. |
Kourda N, Zaraa I, Abid L, Zitouni K, Adouani A, Baltagi-Ben Jilani S, et al. Primary mucinous carcinoma of the skin. A case report. Ann Pathol 2006;26:211-4.
[Google Scholar]
|
| 49. |
Qureshi HS, Salama ME, Chitale D, Bansal I, Ma CK, Raju U, et al. Primary cutaneous mucinous carcinoma: Presence of myoepithelial cells as a clue to the cutaneous origin. Am J Dermatopathol 2004;26:353-8.
[Google Scholar]
|
| 50. |
Paties C, Taccagni GL, Papotti M, Valente G, Zangrandi A, Aloi F. Apocrine carcinoma of the skin. A clinicopathologic, immunocytochemical, and ultrastructural study. Cancer 1993;71:375-81.
[Google Scholar]
|
| 51. |
Smith KJ, Tuur S, Corvette D, Lupton GP, Skelton HG. Cytokeratin 7 staining in mammary and extramammary Paget's disease. Mod Pathol 1997;11:1069-74.
[Google Scholar]
|
| 52. |
Lundquist K, Kohler S, Rouse RV. Intra-epidermal cytokeratin 7 expression is not restricted to Paget cells but is also seen in Toker cells and Merkel cells. Am J Surg Pathol 1999;23:212-9.
[Google Scholar]
|
| 53. |
Goldblum JR, Hart WR. Perianal Paget's disease-a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol 1998;2:170- 9.
[Google Scholar]
|
| 54. |
Hammer A, Hager H, Steiniche T. Prostate-specific antigen-positive extramammary Paget's disease--association with prostate cancer. APMIS 2008;116:81-8.
[Google Scholar]
|
| 55. |
Salamanca J, Benito A, García-Peñalver C, Azorín D, Ballestín C, Rodríguez-Peralto JL. Paget's disease of the glans penis secondary to transitional cell carcinoma of the bladder: A report of two cases and review of the literature. J Cutan Pathol 2004;31:341-5.
[Google Scholar]
|
| 56. |
Wilkinson EJ, Brown HM. Vulvar Paget disease of urothelial origin: A report of three cases and a proposed classification of vulvar Paget disease. Hum Pathol 2002;33:549-54.
[Google Scholar]
|
| 57. |
Kutzner H, Requena L, Rütten A, Mentzel T. Spindle cell predominant trichodiscoma: A fibrofolliculoma/trichodiscoma variant considered formerly to be a neurofollicular hamartoma: A clinicopathological and immunohistochemical analysis of 17 cases. Am J Dermatopathol 2006;28:1-8.
[Google Scholar]
|
| 58. |
Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: A sensitive marker of sebaceous differentiation. Am J Dermatopathol 1999;21:426-31.
[Google Scholar]
|
| 59. |
Liang H, Wu H, Giorgadze TA, Sariya D, Bellucci KS, Veerappan R, et al. Podoplanin is a highly swenetive and specific marker to distinguish primary skin adnexal carcinoma from adenocarcinoma metastatic to skin. Am J Surg Pathol 2007;31:304-10.
[Google Scholar]
|
| 60. |
Ivan D, Hafeez Diwan A, Prieto VG. Expression of p63 in primary cutaneous adnexal neoplasms and adenocarcinoma metastatic to the skin. Mod Pathol 2005;18:137-42.
[Google Scholar]
|
| 61. |
Plumb SJ, Argenyi ZB, Stone MS, De Young BR. Cytokeratin 5/6 immunostaining in cutaneous adnexal neoplasms and metastatic adenocarcinoma. Am J Dermatopathol 2004;26:447- 51.
[Google Scholar]
|
| 62. |
Wallace ML, Longacre TA, Smoller BR. Estrogen and progesterone receptors and anti-gross cystic disease fluid protein 15 (BRST-2) fail to distinguish metastatic breast carcinoma from eccrine neoplasms. Mod Pathol 1995;8:897-901.
[Google Scholar]
|
Fulltext Views
16,479
PDF downloads
6,108





