Translate this page into:
Predisposing factors and histopathological variants of cutaneous squamous cell carcinoma: Experience from a North Indian teaching hospital
2 Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Uma Nahar Saikia
Professor, Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh - 160 012
India
| How to cite this article: Khullar G, Saikia UN, De D, Handa S, Radotra BD. Predisposing factors and histopathological variants of cutaneous squamous cell carcinoma: Experience from a North Indian teaching hospital. Indian J Dermatol Venereol Leprol 2016;82:273-278 |
Abstract
Background: Squamous and basal cell carcinomas together constitute the majority of non-melanoma skin cancers. These malignancies are infrequent in Indians as compared to the white skinned population. Literature on squamous cell carcinoma in dark skin is limited. Aim: To analyze the risk factors and to characterize the histopathological subtypes of cutaneous squamous cell carcinoma in Indian patients in an area, non-endemic for arsenicosis. Methods: A retrospective analysis of data from January 2003 to August 2013 was performed to evaluate the predisposing factors and histopathological types of cutaneous squamous cell carcinoma at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh. Demographic and disease characteristics such as age, gender and predisposing factors, particularly premalignant dermatoses were recorded and histopathology slides were reviewed. Results: Of the 13,426 skin biopsy specimens received during the 10-year period, there were 82 (0.6%) cases of squamous cell carcinoma and 170 (1.7%) of basal cell carcinoma. The mean age at diagnosis of cutaneous squamous cell carcinoma was 53.7 years and the male to female ratio was 2:1. The most common site of involvement was the lower limbs in 34 (41.5%) patients. Marjolin's ulcer was present in 36 (43.9%) cases. No predisposing factor was identified in 35 (42.7%) patients. Histopathologically, the tumors were classified most commonly as squamous cell carcinoma not otherwise specified in 33 (40.2%) cases. Limitations: This was a retrospective study and details of occupation and interval between the precursor lesions and development of tumor were not recorded. Immunohistochemistry for human papilloma virus and p53 tumor suppressor protein were not performed as these tests were not available. Conclusion: Cutaneous squamous cell carcinoma is uncommon in Indian patients and a high index of suspicion is necessary when a rapidly enlarging nodule, verrucous fungating plaque or an ulcer with everted margins develops in long standing scars and other predisposing dermatologic conditions. Histopathological examination is mandatory to confirm the diagnosis and identify the subtype and this has prognostic implications.Introduction
Non-melanoma skin cancers are infrequent in Indians as compared with white skinned populations. Basal cell carcinomas comprise about 75% of skin malignancies in Caucasians, whereas squamous cell carcinomas predominate in dark skinned ethnic groups accounting for 30–65% of cutaneous neoplasms.[1] A study evaluating major dermatologic malignancies in south Nigeria found that squamous cell carcinoma was the most common (37%) of all the skin cancers.[2] In a recent Indian study of non-melanoma skin cancers, squamous cell carcinoma comprised 83.9% and basal cell carcinoma 16.1% of the cases.[3] Ultraviolet radiation is not an important risk factor for squamous cell carcinoma in dark skin owing to the protective effect of eumelanin. Predisposing factors in dark skinned individuals include chronic inflammatory dermatoses such as lichen planus hypertrophicus, genital lichen sclerosus et atrophicus, discoid lupus erythematosus, genodermatoses, cutaneous infections, non-healing ulcers, long standing scars, and immunosuppression. As the latent period for malignant transformation is often prolonged, dermatologists should be vigilant in patients with these premalignant dermatoses, schedule regular follow-ups and consider histopathologic examination on clinical suspicion to avoid misdiagnosis or delayed diagnosis. Data regarding the potential risk factors for cutaneous squamous cell carcinoma pertaining to Indian skin is largely limited to case reports. This study is a retrospective evaluation of the predisposing factors and histopathological variants of cutaneous squamous cell carcinoma in Indian patients over a period of 10 years.
Methods
A retrospective analysis of all the cases of cutaneous squamous cell carcinoma diagnosed histopathologically at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh over 10 years from 2003 to 2013 was conducted. The demographic profile and predisposing factors with specific reference to primary dermatoses were recorded from the hospital database. The histopathology slides were reviewed by two observers (UNS and GK) to determine the type of squamous cell carcinoma.
Results
A total of 13,426 skin biopsy specimens were reported in our histopathology department during the 10-year period, of which 82 (0.6%) were squamous cell carcinomas and 170 (1.7%) were basal cell carcinomas. The majority of cases were seen by dermatologists at presentation, while some of the Marjolin's ulcers were seen by the plastic surgery services. The mean age at diagnosis of cutaneous squamous cell carcinoma was 53.7 years (range 25–95 years) and males outnumbered females in the ratio of 2:1. The most frequent site of involvement was the lower limbs (34 cases, 41.5%), followed by head and neck (25 cases, 30.5%), upper limbs, genitalia, and trunk [Table - 1].

Squamous cell carcinomas developed in chronic scars and wounds (Marjolin's ulcer) in 36 (43.9%) cases. Most of these patients had post-burn scars [Figure - 1], while a few had chronic non-healing ulcers, chronic osteomyelitis with discharging sinuses and post-surgical scar [Table - 2]. Squamous cell carcinomas arose in chronic inflammatory dermatoses and genodermatoses in 10 (12.2%) patients; these included genital lichen sclerosus et atrophicus in 4 (4.9%) patients [Figure - 2]a, verrucous discoid lupus erythematosus [Figure - 2]b in 3 (3.7%), lichen planus hypertrophicus [Figure - 3]a in 2 (2.4%), and xeroderma pigmentosum [Figure - 3]b in 1 (1.2%) patient. Post-renal transplant immunosuppression was suspected to be the cause in one case (1.2%). No predisposing factors were found in 35 (42.7%) patients [Table - 2].
 |
| Figure 1: Ulcer with raised, indurated, pigmented margins and clean erythematous floor overlying a burn scar on upper back |
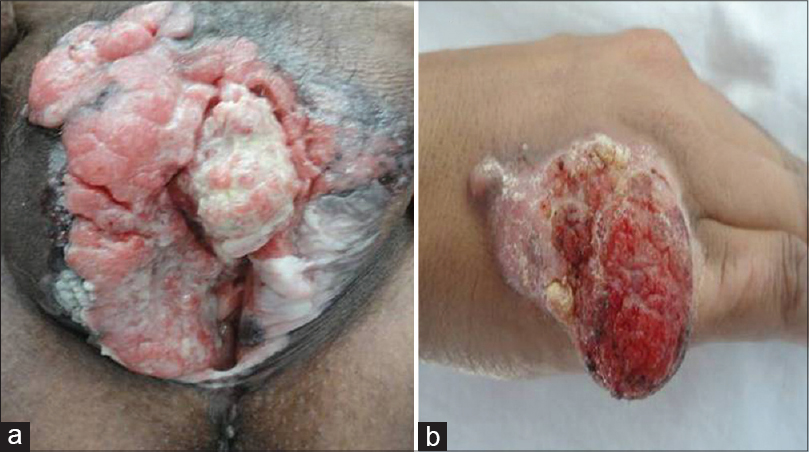 |
| Figure 2: (a) Fungating growth covered with yellowish slough arising in lichen sclerosus of vulva, (b) Verrucous growth with areas of ulceration overlying a plaque of hypertrophic discoid lupus erythematosus on the dorsum of hand |
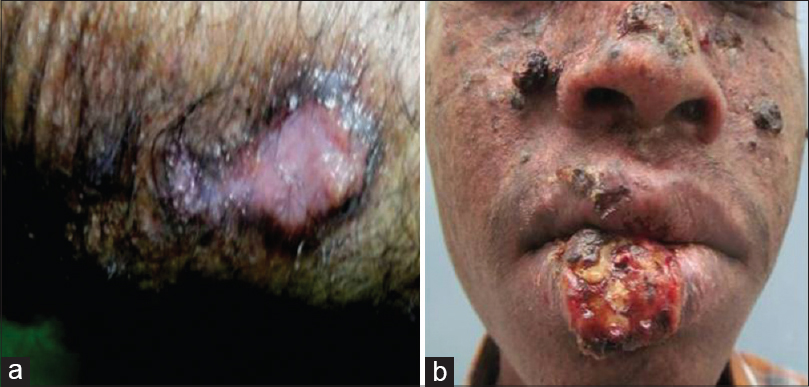 |
| Figure 3: (a) Fleshy nodule on the inferior border of lichen planus hypertrophicus on the forearm, (b) Ulcer with erythematous base, raised margins and overlying yellowish-black crust on the lower lip in xeroderma pigmentosum |
The age-wise distribution of predisposing conditions for cutaneous squamous cell carcinoma is shown in [Table - 3]. Predisposing conditions were common (29/34; 85.3%) among tumors developing on the lower limbs, with Marjolin's ulcers appearing in chronic scars in 27 cases, and verrucous discoid lupus erythematosus and lichen planus hypertrophicus in 1 case each. The remaining five patients had de novo squamous cell carcinoma, of whom three were farmers and two were government employees.
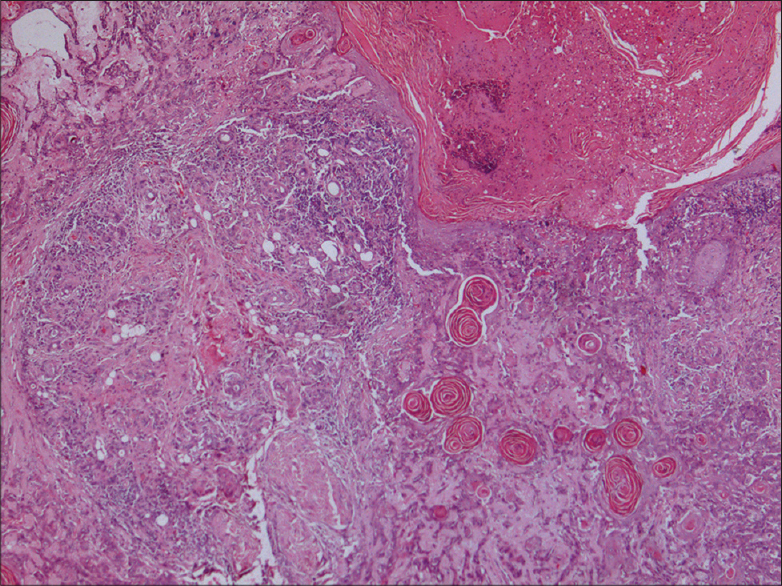
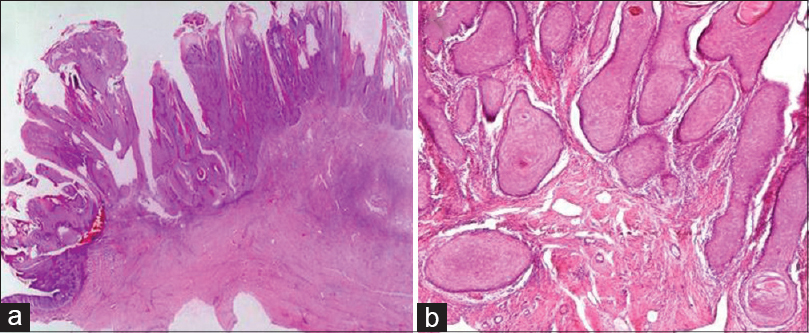
The two most common histopathological subtypes of cutaneous squamous cell carcinoma were not otherwise specified in 33 (40.2%) and keratinizing [Figure - 4] in 30 (36.6%) patients. Less frequent histopathological subtypes seen were poorly differentiated [Figure - 5], verrucous [Figure - 6] and spindle cell [Figure - 7] in 4 (4.9%) cases each, moderately differentiated in 3 (3.7%), basaloid in 2 (2.4%), adenosquamous [Figure - 8]a and acantholytic [Figure - 8]b in 1 (1.2%) case each [Table - 4].
 |
| Figure 4: Well-differentiated squamous cell carcinoma (H and E, ×40) |
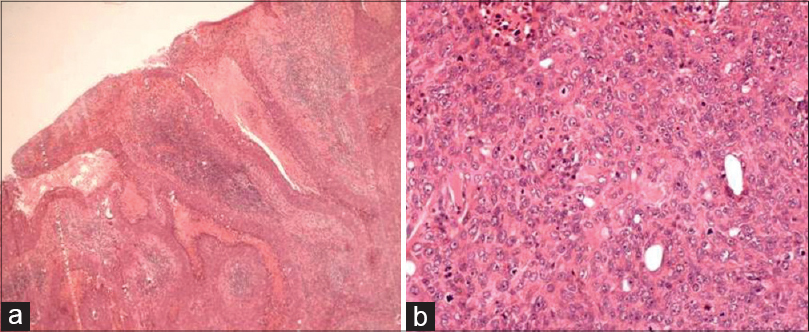 |
| Figure 5: (a) Poorly differentiated squamous cell carcinoma showing ulceration (H and E, ×40), (b) Atypical cells showing vesicular nuclei, prominent nucleoli, and bizarre mitosis (H and E, ×100) |
 |
| Figure 6: (a) Verrucous carcinoma showing papillomatosis (H and E, ×20), (b) Bulbous downgrowths invading into the dermis (H and E, ×100) |
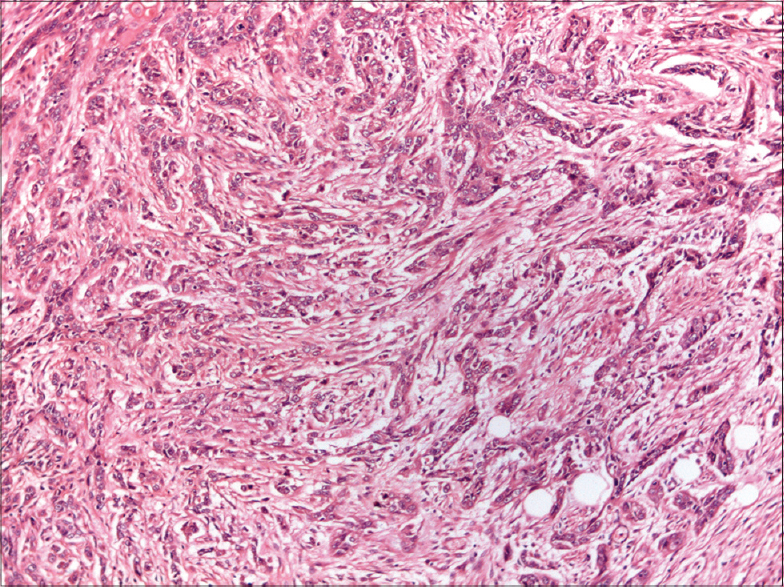 |
| Figure 7: Spindle cell variant showing tumor cells interspersed in desmoplastic stroma (H and E, ×100) |
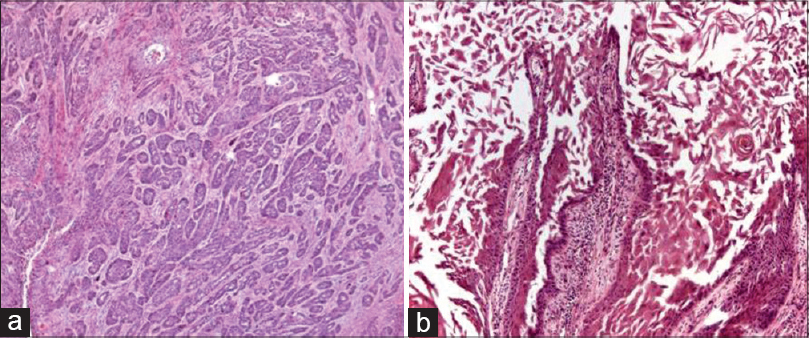 |
| Figure 8: (a) Tumor cells forming tubules and acini in adenosquamous variant of squamous cell carcinoma (H and E, ×100), (b) Acantholytic variant depicting loss of cohesion of keratinocytes resulting in central pseudoglandular appearance with tombstoning of cohesive cells in the periphery (H and E, ×100) |
Discussion
Malignant skin neoplasms contribute to 20–30% of all malignancies in Caucasians but only 2-4% in Asians, and 1–2% in blacks and Indians.[1] Dark skinned individuals produce more melanin and have larger and more dispersed melanosomes which filter twice as much ultraviolet radiation as the smaller and clustered melanosomes in Caucasian skin, thereby protecting against cutaneous malignancies. In Blacks and Asian Indians, squamous cell carcinoma is the most common cutaneous neoplasm, accounting for 30–65% of the cases whereas in white skin squamous cell carcinoma ranks second to basal cell carcinoma, representing 15–25% of cutaneous malignancies.[1] Interestingly, we found basal cell carcinoma to be twice as common as squamous cell carcinoma. This is similar to the findings of another Asian study on non-melanoma skin cancers, wherein basal cell carcinoma was reported in 62.2% and squamous cell carcinoma in 37.8% patients.[4] The mean age at diagnosis of cutaneous squamous cell carcinoma in our was 53.7 years (range 25–95 years) with a male to female ratio of 2:1. Adinarayan et al.[3] reported a similar peak age in the incidence of squamous cell carcinoma with 50% cases falling in the age group of 41–60 years and a male to female ratio of 3.3:1. In the dark skin phenotype, cutaneous squamous cell carcinoma most frequently presents on sun-protected sites suggesting that ultraviolet radiation may have a smaller role in its induction.[1]
The most common site of involvement in our study was the lower limbs (41.5%), followed by head and neck;[6] however in a recent study on non-melanoma skin cancers from Karnataka [3] the head and neck (50%) was the most frequently involved site, followed by the lower limbs (38.4%). In a study evaluating dermatologic malignancies in Nigeria, the lower limb was the most commonly affected site, followed by external genitalia and anus.[2] The predilection of cutaneous squamous cell carcinoma for a relatively photoprotected site such as the lower limbs in our study may be due to the frequent occurrence of predisposing lesions (burn scars, chronic non-healing ulcers and lichen planus hypertrophicus) at this site.
It is important that dermatologists should be aware of the underlying risk factors for cutaneous squamous cell carcinoma for early diagnosis and appropriate treatment. Cutaneous squamous cell carcinoma in dark skin tends to arise in non-healing ulcers, chronic scars, inflammatory, infectious, and genodermatoses in 20–40% of cases.[1] In the present study, post-burn scars (32.9%) and chronic non-healing ulcers (8.5%) were the most frequent precursor lesions for Marjolin's ulcer.[5] Marjolin's ulcer develops at sites of chronic irritation or injury and although burn scars are the most common precursor lesions, they may also arise in pressure sores, venous ulcers, traumatic wounds, osteomyelitis, sinus tracts and fistulas. In a review of 433 patients with Marjolin's ulcer, the lower limb was the most common site of involvement in 53.3% cases and the most frequent predisposing factor was burn scar (76.5%), followed by traumatic wounds (8.1%).[6]
Chronic inflammatory dermatoses and genodermatoses were identified as a predisposing factor in 12.2% cases in our study.[5] Genital lichen sclerosus et atrophicus was the most common premalignant dermatosis, affecting three women and one man in this study. The risk of developing squamous cell carcinoma in vulvar lichen sclerosus et atrophicus is 3–7%, with almost 60% of vulvar squamous cell carcinoma showing histological features of lichen sclerosus et atrophicus. In penile lichen sclerosus et atrophicus, the risk for squamous cell carcinoma varies from 4% to 8%, with 32–50% of patients of penile squamous cell carcinoma (SCC) having evidence of lichen sclerosus et atrophicus on biopsy.[7]
Three patients in our study developed squamous cell carcinoma overlying verrucous discoid lupus erythematosus, -two had lesions on the dorsum of the hand and one on the leg. Malignant transformation of discoid lupus erythematosus may occur in 3.3% cases with the interval between the diagnosis of discoid lupus erythematosus and malignant transformation ranging from 26 to 41 years (mean 30.8 years).[8]
Squamous cell carcinoma developed in lichen planus hypertrophicus on the forearm and leg in two patients. Malignant transformation of cutaneous lichen planus is rare (0.4%) with more than 56% cases arising in lichen planus hypertrophicus;[9] it may also complicate 0.3–3% cases of oral lichen planus, of which 40–60% are erosive forms.[10]
Only one patient in our study had underlying xeroderma pigmentosum.[5] Basal cell carcinoma, squamous cell carcinoma and lentigo maligna melanoma are the most frequent tumors arising in xeroderma pigmentosum with the risk of cutaneous malignancy increased 1000-fold at less than 20 years of age.
Other inflammatory and infectious dermatoses such as erythema ab igne, porokeratosis, hidradenitis suppurativa, donovanosis, lupus vulgaris, and less frequently genodermatoses like epidermodysplasia verruciformis and epidermolysis bullosa may give rise to squamous cell carcinoma in dark skin.[5]
Histopathologically, squamous cell carcinoma is broadly classified into well, moderately, and poorly differentiated types. The two most common histological subtypes in the present study were not otherwise specified (40.2%) and keratinizing/well-differentiated squamous cell carcinoma (36.6%). Moderately and poorly differentiated subtypes were recognized in 3.7% and 4.9% cases, respectively.[5] A study analyzing clinico-histopathological aspects of non-melanoma skin cancers from India described 46% of squamous cell carcinomas as well-differentiated, 43.2% as moderately differentiated and 11.5% as poorly differentiated.[3] Cutaneous squamous cell carcinoma has been classified with respect to malignant potential into four categories: high (>10% metastatic rate), intermediate (3–10%), low (≤2%), and indeterminate.[11] High risk variants include squamous cell carcinoma arising de novo, complicating burn scar, radiation and immunosuppression, invasive Bowen's disease, adenosquamous and malignant proliferating pilar tumors. Acantholytic, intraepidermal epithelioma with invasion and lymphoepithelioma-like carcinoma of the skin comprise tumors with intermediate behavior while those developing in actinic keratosis, human papilloma virus associated, tricholemmal carcinoma and spindle cell squamous cell carcinoma (unassociated with radiation) fall into one low risk category.
Verrucous carcinoma is a low grade, well differentiated variant of squamous cell carcinoma. Although any site may be involved, it is commonly described in the anogenital region (Buschke–Lowenstein tumor), oral mucosa, and plantar surface of the foot (carcinoma cuniculatum).[5] Of the four patients with verrucous carcinoma in our study, two presented with lesions on the sole and one each on the glans penis and face.
Spindle cell or sarcomatoid squamous cell carcinoma is a rare variant that shows atypical spindle cells with elongated vesicular nuclei forming fascicles that infiltrate singly, deep into the dermis and subcutis. Characteristically, it is positive for both cytokeratins and vimentin. The spindle cell variant was recognized in four cases in our study. A de novo presentation of this variant has been reported from India as a painful firm nodule with sinuses discharging blackish material on the face.[12]
Basaloid squamous cell carcinoma has been mostly described in the aerodigestive tract and rarely in the skin. Immunohistochemical stains reveal positivity for cytokeratins and negativity for Ber-EP4.[13] Two of our patients were diagnosed with the basaloid variant.
Adenosquamous carcinoma is a rare variant considered to be more aggressive than the classical type. It is characterized by anastomosing islands of squamous cells with formation of keratin pearls and glands lined by a cuboidal or low columnar epithelium. Glandular differentiation is highlighted by carcinoembryonic antigen positivity.[14] This subtype was found in one patient in our study.
Acantholytic or adenoid variant is histologically characterized by typical squamous differentiation with areas of keratinocyte acantholysis giving a pseudoglandular appearance and has a favorable prognosis. We recorded only one patient with acantholytic variant of squamous cell carcinoma.
The limitation of our study was retrospective assessment of records due to which complete clinical details including occupational history and interval between the precursor lesion and onset of malignancy could not be ascertained. We could not perform immunohistochemistry for human papilloma virus and p53 tumor suppressor protein due to unavailability of these facilities.
Conclusion
The present study highlights the common dermatologic conditions that can evolve into squamous cell carcinoma and the spectrum of histopathologic variants. As the prognosis of squamous cell carcinoma in the setting of premalignant dermatoses is poor, such patients should be periodically followed up and subjected to histopathological examination for definite diagnosis, thereby facilitating timely intervention.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol 2006;55:741-60.
[Google Scholar]
|
| 2. |
Asuquo ME, Ngim O, Ugare G, Omotoso J, Ebughe G. Major dermatologic malignancies encountered in a teaching hospital surgical department in South Nigeria. Am J Clin Dermatol 2008;9:383-7.
[Google Scholar]
|
| 3. |
Adinarayan M, Krishnamurthy SP. Clinicopathological evaluation of nonmelanoma skin cancer. Indian J Dermatol 2011;56:670-2.
[Google Scholar]
|
| 4. |
Alakloby OM, Bukhari IA, Shawarby MA. Histopathological pattern of Non-melanoma skin cancer at King Fahd Hospital of the University in the Eastern Region of Saudi Arabia during the years 1983 to 2002. Cancer Ther 2008;6:303-6.
[Google Scholar]
|
| 5. |
Khullar G, Saikia UN, De D, Radotra BD. Nonmelanoma skin cancers: An Indian perspective. Indian J Dermatopathol Diagn Dermatol 2014;1:55-62
[Google Scholar]
|
| 6. |
Kerr-Valentic MA, Samimi K, Rohlen BH, Agarwal JP, Rockwell WB. Marjolin's ulcer: Modern analysis of an ancient problem. Plast Reconstr Surg 2009;123:184-91.
[Google Scholar]
|
| 7. |
Gutiérrez-Pascual M, Vicente-Martin FJ, Lopez-Estebaranz JL. Lichen sclerosus and squamous cell carcinoma. Actas Dermosifiliogr 2012;103:21-8.
[Google Scholar]
|
| 8. |
Millard LG, Barker DJ. Development of squamous cell carcinoma in chronic DLE. Clin Exp Dermatol 1978;3:161-6.
[Google Scholar]
|
| 9. |
Sigurgeirsson B, Lindelof B. Lichen planus and malignancy. An epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol 1991;127:1684-8.
[Google Scholar]
|
| 10. |
Castano E, Lopez-Rios F, Alvarez-Fernandez JG, Rodriguez- Peralto JL, Iglesias L. Verrucous carcinoma in association with hypertrophic lichen planus. Clin Exp Dermatol 1997;22:23-5.
[Google Scholar]
|
| 11. |
Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: A Comprehensive clinicopathologic classification. Part One. J Cutan Pathol 2006;33:191-206.
[Google Scholar]
|
| 12. |
Panda S. Nonmelanoma skin cancer in India: Current scenario. Indian J Dermatol 2010;55:373-8.
[Google Scholar]
|
| 13. |
Boyd AS, Stasko TS, Tang YW. Basaloid squamous cell carcinoma of the skin. J Am Acad Dermatol 2011;64:144-51.
[Google Scholar]
|
| 14. |
Fu JM, McCalmont T, Yu SS. Adenosquamous carcinoma of the skin: A case series. Arch Dermatol 2009;145:1152-8.
[Google Scholar]
|
Fulltext Views
6,772
PDF downloads
3,338





