Translate this page into:
Premature graying of hair
2 Department of Dermatology, Chacha Nehru Bal Chikitsalaya, Delhi, India
Correspondence Address:
Deepika Pandhi
Department of Dermatology and STD, University College of Medical Sciences and Associated Guru Teg Bahadur Hospital, University of Delhi, Delhi
India
| How to cite this article: Pandhi D, Khanna D. Premature graying of hair. Indian J Dermatol Venereol Leprol 2013;79:641-653 |
Abstract
Premature graying is an important cause of low self-esteem, often interfering with socio-cultural adjustment. The onset and progression of graying or canities correlate very closely with chronological aging, and occur in varying degrees in all individuals eventually, regardless of gender or race. Premature canities may occur alone as an autosomal dominant condition or in association with various autoimmune or premature aging syndromes. It needs to be differentiated from various genetic hypomelanotic hair disorders. Reduction in melanogenically active melanocytes in the hair bulb of gray anagen hair follicles with resultant pigment loss is central to the pathogenesis of graying. Defective melanosomal transfers to cortical keratinocytes and melanin incontinence due to melanocyte degeneration are also believed to contribute to this. The white color of canities is an optical effect; the reflection of incident light masks the intrinsic pale yellow color of hair keratin. Full range of color from normal to white can be seen both along individual hair and from hair to hair, and admixture of pigmented and white hair is believed to give the appearance of gray. Graying of hair is usually progressive and permanent, but there are occasional reports of spontaneous repigmentation of gray hair. Studies evaluating the association of canities with osteopenia and cardiovascular disease have revealed mixed results. Despite the extensive molecular research being carried out to understand the pathogenesis of canities, there is paucity of effective evidence-based treatment options. Reports of repigmentation of previously white hair following certain inflammatory processes and use of drugs have suggested the possibility of cytokine-induced recruitment of outer sheath melanocytes to the hair bulb and rekindled the hope for finding an effective drug for treatment of premature canities. In the end, camouflage techniques using hair colorants are outlined.Introduction
Skin and hair contribute immensely in human communication. Hair length, color, and style play an important role in people′s physical appearance and self-perception. Human beings are unique amongst primates in having very thick, long, and highly pigmented scalp hair. This is likely to have provided one or more survival benefits to the humans during the process of evolution. Firstly, selective and avid binding of toxins and metals to melanin pigment aids in preventing the buildup of toxic metals from fish species which concentrate heavy metals, especially in human development along sea coasts and riverbanks. [1] Secondly, reactive quinone intermediates generated during melanin synthesis possess potent antibacterial properties. Lastly, deep brown-black hair present in 90% of the world′s population protects against sunstroke, and its melanin aids very efficient and fast exchange of ion transport and efflux for adequate salt balance. [1] However, the remaining 5-10% of world population mostly hailing from northern Europe do not have the environment-friendly brown-black hair, possibly due to mutations in the melanocortin-1 receptor (MC1R), a G-protein coupled receptor. Mutations in the MC1R gene are believed to have contributed to white blonde, yellow blonde, and auburn color of hair in individuals in the less sunny climates in northern Europe, while natural selection pressures possibly restrained this mutation in the sunny tropical areas. [2],[3]
Considering the important role played by hair in social communication, premature hair graying or canities has significant adverse effects on the appearance, self-esteem, and socio-cultural acceptance of the affected individual. It is often viewed as a sign of old age and loss of health and vigor. Affected individuals are often subjected to social stigma, discrimination, and difficulties in marriage.
Definition
Canities, or hair graying, is a process of chronological aging and occurs regardless of gender or race. The age of graying varies with race and ethnicity. Hair is said to gray prematurely only if graying occurs before the age of 20 years in Whites, before 25 years in Asians [Figure - 1] and [Figure - 2], and before 30 years in Africans. [4]
 |
| Figure 1: A 10-year old girl with premature canities |
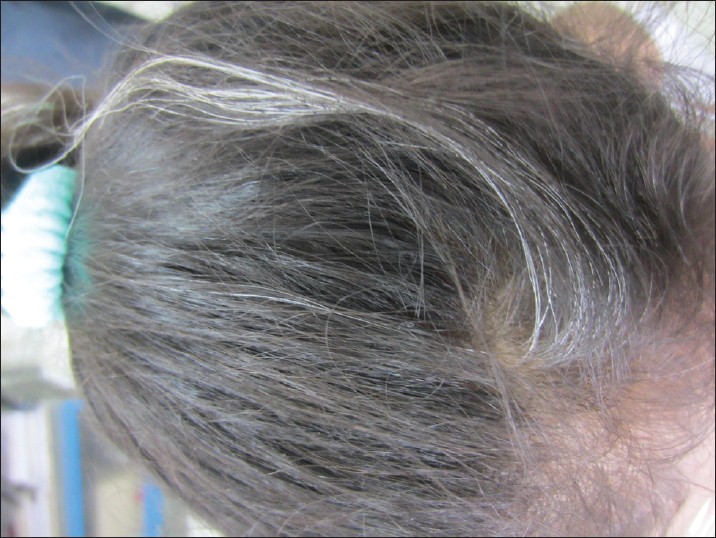 |
| Figure 2: Streak of gray-white hair in a 7-year-old child without associated vitiligo |
Normal Hair Follicular Melanin Unit and Melanogenesis
The color of human hair depends on melanogenesis, the process of synthesis of melanin and its subsequent distribution from the melanocyte to keratinocyte. The process is thought to be regulated genetically at various levels. The human hair follicles contain two types of melanins: the black-brown pigment eumelanins mainly present in black and brown hair and the yellow or red pheomelanins in auburn and blonde hair. [4]
Both epidermal and follicular melanocytes are derived from immature melanoblasts that migrate from the neural crest into the skin during embryogenesis. As the hair follicle develops, the progeny of melanoblasts which proliferate in the epidermis, known as transient-amplifying melanocytes, leave that compartment and move into the developing hair follicle. There, melanocytes may become or remain DOPA-oxidase-positive cells (i.e. express active tyrosinase) or remain DOPA-oxidase-negative cells (i.e. either fail to express tyrosinase or express an inactive tyrosinase) depending on the intrafollicular compartment in which they reside [Figure - 3]. [5],[6]
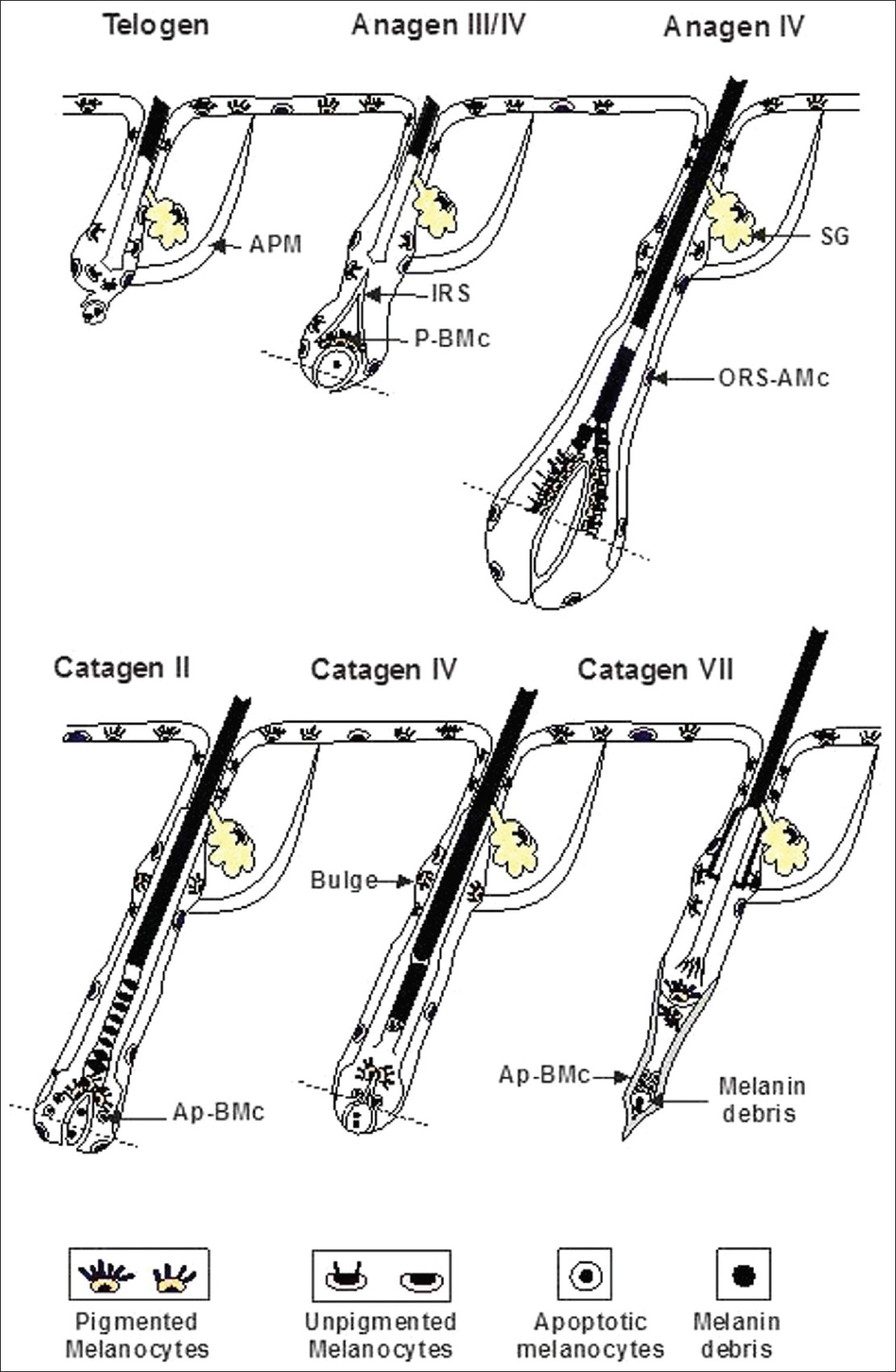 |
| Figure 3: Melanocyte stem cells and their progeny during the hair cycle (APM: Arrector pilorum muscle, SG: Sebacean gland, IRS: Inner root sheeth, P.BMc: Pigmented hair bulb melanocytes, ORS-AMC: Outer root apoptotic melanocytes, Ap-BMc: Apoptotic hair bulb melanocytes) |
The hair follicle melanin unit consists of one melanocyte to five keratinocytes in the hair bulb as a whole and one to one in the basal layer of the hair bulb matrix. By contrast, each epidermal melanocyte is associated with 36 viable keratinocytes in the immunocompetent epidermal-melanin unit. Hair follicle melanogenesis is under cyclical control and tightly coupled to the hair growth cycle, in contrast to epidermal melanogenesis that is continuous. Hair growth has three periods: hair shaft production (anagen), brief apoptosis driven regression phase when the lower two-thirds of hair follicle are resorbed (catagen), and a relatively quiescent period (telogen). Melanocytes in the hair bulb retract their melanocytes and shut down melanogenesis towards the end of anagen. Simultaneously there is a decline in the activity of three main melanogenic enzymes: tyrosinase, gp75, and dopachrome tautomerase. [4] This occurs a few days before the cessation of keratinocyte proliferation resulting in the pigment-free proximal ends of shed telogen hair. During catagen, hair apoptosis occurs and a quiescent hair follicle much smaller in size is left in telogen. Melanogenic activity restarts during early anagen with the reconstruction of the follicular melanin unit. Tyrosinase activity becomes apparent during anagen III, pigment transfer from hair bulb melanocytes to cortical keratinocytes is initiated during anagen IV and active melanogenesis continues throughout anagen V and VI, ceasing with the onset of catagen. [4],[7] Anagen usually persists for 3-5 years, and these follicles extrude the hair fiber at a rate of approximately 1 cm per month. [8] Melanocytes are present in two compartments of the hair follicle: in the anagen hair bulb, where they transfer pigment to cells that will form the hair cortex, and in the outer root sheath (ORS). ORS melanocytes are sparsely distributed in the basal layer of the epithelium along the length of the follicle and are non-melanized. However, recent studies suggest that gray hair follicles lack melanocytes in the hair bulb while retaining those in the ORS. [7] Hair bulb melanocytes are probably recruited from the ORS melanocyte population at the onset of anagen. Migration and activation of these melanocytes is possibly under unknown local signalling mechanisms like α- melanocyte stimulating hormone (α-MSH); modulation or failure of which may result in graying. [7] The hair bulb matrix is the principal site for the fully differentiated follicular melanocyte subpopulation; these melanocytes are distributed, in particular, within the matrix above and around the upper dermal papilla. They transfer their melanin granules to keratinocytes of the hair cortex and less so to the medulla and very rarely to the hair cuticle. [9] Under stimulation from radiation or cytokines, the ORS melanocytes may be stimulated to migrate and differentiate to naturally repigment graying hair follicles. [4]
Age-Related Changes in the Hair Follicle Melanin Unit
Different types of hair fibers produced during life include: fine unpigmented lanugo hair in the fetus or neonate, short (mostly unpigmented) vellus hair or fine pigmented intermediate hair and long thick terminal hair shafts in the adult. The surface morphology of hair also appears to change with age, most particularly with the reduction in the cuticular scale size. Hair color on the scalp tends to darken with advancing age. [10] The hair bulb melanocytes have high synthetic capacity that is greatest during youth when the scalp follicular melanin unit is only a few cycles old. An average scalp hair follicle usually receives 7 ± 15 melanocyte replacements from an ORS reservoir to the hair bulb in 45 years preceding onset of gray hair. [4] Senile canities are believed to occur because of exhaustion of the regenerative capacity of hair pigmentation as well as through programmed events during aging.
Premature graying or canities may reflect a genetically regulated early exhaustion of the melanocyte reservoir′s seeding potential or some defect in cell activation/migration compounded by environmental factors, inflammation, or psychological stress. Nishimura et al. suggested that loss of melanocyte stem cells can be observed and temporarily precedes the loss of differentiated melanocytes in the hair matrix. This incomplete maintenance of melanocyte stem cells appears to cause physiologic hair graying through loss of differentiated progeny with aging. [11] The progression of graying is compounded by the fact that with advancing age more hair follicles remain for longer duration in the resting phase (telogen). [4]
Histopathology of Canities
A line across the widest part of the bulb of the hair follicle divides it into two regions: a lower region of undifferentiated cells and an upper region in which the cells become differentiated to form the inner sheath and the hair [Figure - 4]. Below this critical level known as the line of Auber lie the matrix or the germination center of the follicle, where every cell is mitotically active, and the dermal papilla. From the matrix, cells move to the upper part of the bulb, where they increase in volume and become elongated vertically. Some of the cells in the upper bulb still show some mitotic activity, but these are too few to account for much of the growth of the hair. The pigmentary unit is a pear-shaped black structure at the tip of the dermal papilla above the Auber′s line in pigmented hair where individual melanocytes cannot be distinguished. [12] Only unpigmented and undifferentiated putative melanocyte stem cells, but not pigmented differentiated melanocytes, are normally found in the hair bulb below the line of Auber. In gray hair, the pigmentary unit becomes fuzzy, melanocytes are few and rounded, and lightly pigmented oligodendritic melanocytes become detectable in the proximal hair bulb below Auber′s line. [13] The resultant pigment loss in graying hair follicles due to a marked reduction in melanogenically active melanocytes in the hair bulb of gray anagen hair follicles is central to the pathogenesis of graying. [6] Defective melanosomal transfer to the cortical keratinocytes or melanin incontinence due to melanocyte degeneration is also believed to contribute to graying. Ultrastructurally, remaining melanocytes contain fewer and smaller melanosomes. In addition, there is autophagolysosomal degradation of melanosomes within the melanocytes itself and is usually followed by the degeneration of the melanocyte. Degenerative changes within the hair follicle melanocytes are associated with the parallel increase in dendritic cells in the anagen hair follicle. [4] Eventually, no melanogenic melanocytes remain in the hair bulb. True gray hair show reduced DOPA reaction (indicator of tyrosinase activity) while white hair bulbs are negative for the same. [4]
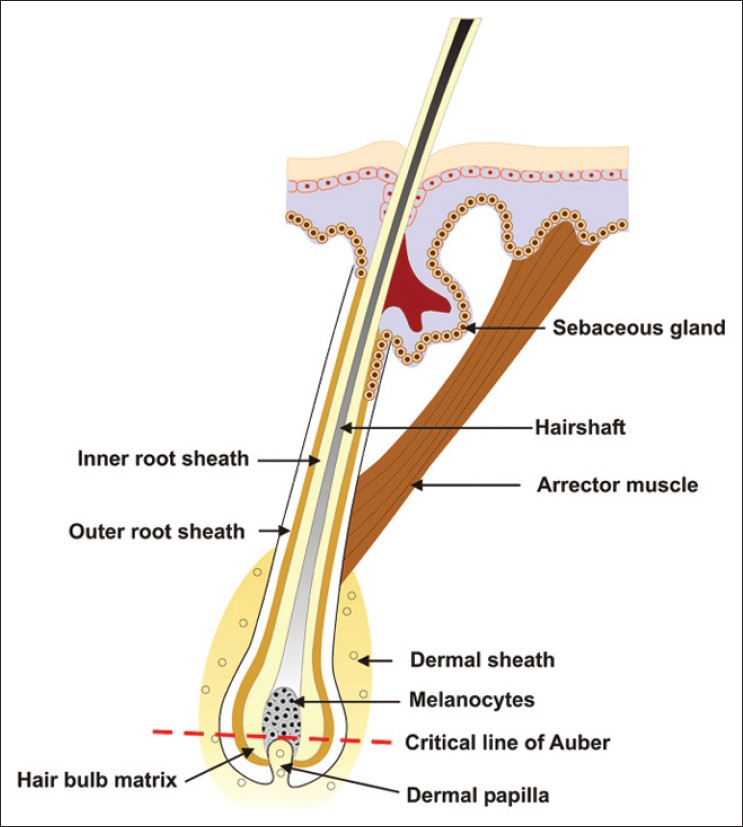 |
| Figure 4: Pigmentary unit in relation to the dermal papillae and the line of Auber |
Pathogenesis of Canities
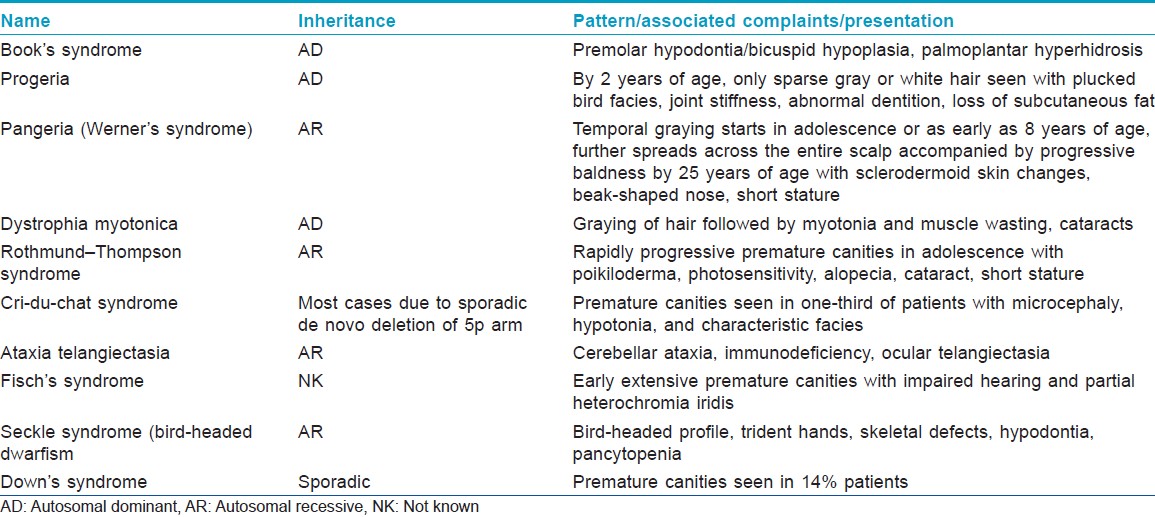
As of now, the etiology of graying is incompletely understood. Currently, it is mainly considered to be genetic with interplay of various environmental factors . Premature canities may appear alone without any underlying pathology as an autosomal dominant condition occurring before 20 years of age. It may also occur in association with certain organ-specific autoimmune disorders like pernicious anemia, hyper- or hypothyroidism, and as part of various premature aging syndromes (e.g. progeria and pangeria) [Table - 1] and atopic diathesis. [14],[15] Fifty-five percent of patients with pernicious anemia were found to develop graying of hair before 50 years, as compared to only 30% in the control group. [16] Reversible hypopigmentation of hair has also been noted in association with nutritional deficiencies like chronic protein loss (due to kwashiorkor, nephrosis, celiac disease, and other causes of malabsorption), severe iron deficiency and copper deficiency. [14] Serum copper was significantly lower in 66 patients with premature canities, as compared to normal controls (66) in one study. However, no difference was found in the zinc levels, while iron concentration was higher in the control group. [17] Binding of copper ions to the enzyme tyrosinase, which is essential for enzyme activity and thus the process of melanogenesis, may possibly be affected in patients with premature canities and low serum copper levels. [17]
Other causes implicated include stress, and administration of certain drugs including chloroquine, mephenesin, phenylthiourea, triparanol, fluorobutyrophenone, dixyrazine, the epidermal growth factor receptor inhibitor imatinib and interferon - alpha, and use of certain chemicals (medicated oils) and topically applied agents like dithranol, chrysarobin, resorcin, prostaglandin F 2 alpha (PGF 2 alpha) analogs. [14],[18],[19] Premature graying has also been reported in patients with HIV infection, cystic fibrosis, and Hodgkin′s lymphoma. [18],[20],[21]
The extraordinary melanogenic activity of pigmented bulbar melanocytes in the growing (anagen) hair follicle, continuing for up to 10 years in some hair follicles generates large amounts of reactive oxygen species (ROS) via the hydroxylation of tyrosine and the oxidation of DOPA to melanin and places melanocytes under a higher oxidative stress load. Impairment of antioxidant system with age probably leads to accumulation of ROS and oxidative stress that damages the melanocyte. [25] Wood et al. demonstrated catalase and methionine reductase A and B expression and functional loss of methionine sulfoxide repair mechanism in the gray hair follicle. [26] Oxidative stress generated outside hair follicle melanocytes, for example, by pollution, UV light, psycho-emotional or inflammatory stress, may add to this endogenous oxidative stress and overwhelm the hair follicle melanocyte antioxidant capacity resulting in enhanced terminal damage in the aging hair follicle. [4],[13],[27],[28],[29],[30],[31],[32] Superoxide radicals generated by interaction of UV-A light with topically applied psoralen have recently been shown to induce graying of hair in mice. This photosensitization induced graying was, however, averted by pre-treatment with superoxide dismutase gel on the opposite side. [28] Bcl-2 is an antioxidative stress protein required for maintenance of hair follicle melanocytes at the tip of hair bulb and lack of this protein is associated with disappearance of melanocyte stem cell precursors. [11],[13]
Alternatively, some authors have suggested that the primary cause of hair graying may be associated with hair growth patterns, hair growth rate, or anagen prolongation in the hair cycle, and active hair growth makes conditions less favorable for melanocyte survival in the hair follicle. [33],[34],[35]
Apart from oxidative stress, other factors may also contribute to the process of graying. Insufficient neuroendocrine stimulation of hair follicle melanogenesis by locally synthesized agents, such as adrenocorticotrophic hormone, α-MSH, and β-endorphin, have also been hypothesized as a possible mechanism for hair graying. [36],[37] It has been suggested that binding sites for the pro-eumelanogenic peptide α-MSH are only expressed on melanogenically active melanocytes in pigmented hair follicles and their absence in senile white hair melanocytes may render these cells unresponsive to the melanogenic influence of this melanotrophin. [38] Cervical and lumbar sympathectomy of long duration has also been shown to retard the normal graying of scalp and pubic hair, respectively, in two patients, suggesting that sympathetic denervation somehow slows or prevents the normal graying of hair with increasing age. [39],[40]
Smoking was reported to be significantly correlated with hair graying, and impairment of stem cell regenerative capacity with substance abuse was postulated to lead to graying in a single case report. [41],[42] Interestingly, absence of graying of hair over the pinna in the presence of physiologic canities over the scalp, beard, and moustache regions was reported in 250 Indian men over the age of 50 years. The authors suggested the possibility of a Y-linked ethnic trait that may have a control on the retention of pigment. [43]
Extensive research in the field of premature graying of hair is underway at the molecular level. Bmpr2, a known receptor for bone morphogenetic proteins (Bmps), and Acvr2a, a known receptor for Bmps and activins, are individually redundant but together essential for multiple follicular traits. Reduced Bmpr2/Acvr2a function in melanocytes in mutant mice was recently shown to result in gray hair due to aberrant hair shaft and melanosomes′ differentiation. [44] Stem cell factor (SCF) and its receptor (KIT) were shown to have an important role in signaling in the maintenance of human hair follicle melanogenesis during the anagen cycle and in physiological aging of the hair follicle pigmentary unit. [45] Both Notch 1 and Notch 2 signaling pathways are required for the maintenance of melanoblasts and melanocyte stem cells and are essential for proper hair pigmentation in mice. [46]
Epidemiology
As the age of onset of canities is dependent more on the genotype of the individual, it is subject to racial variation. Average age of onset in Caucasians is 34 ± 9.6 years, and in Negroes, it is 43.9 ± 10.3 years. [14] Graying of hair appears between 30 and 34 years in Japanese men and between 35 and 39 years in Japanese women. [14] On an average, Caucasians begin to gray in their mid-30s, Asians in their late 30s, and Africans, latest in their mid-40s. [4] In Bantus, graying of hair is said to be uncommon before 40-50 years of age. [47] However, onset as early as the second decade or as late as the ninth decade is also possible. [38] A recent study reported that 6-23% of people have 50% gray hair by 50 years of age. [48] Graying is more readily apparent and noticed earlier in those with dark hair, but fair-haired individuals appear totally gray earlier. [22] Both sexes are equally affected. [38]
Clinical Features
The hair follicle pigmentary unit is maximally functioning during post-adolescence and early adulthood, when terminal hair growth is optimal and hair color has settled to its preferred tonal variant. The onset and progression of graying correlate very closely with chronological aging and at least a few gray hair are found in all individuals regardless of gender or race by 60 years of age. [49] In contrast to aging of our skin, premature graying may not be hastened by cumulative photodamage. [9]
In men, graying usually begins at the temples and in the sideburns. Later it spreads to the vertex and the remainder of the scalp, affecting the occiput last. Women usually start graying around the perimeter of the hairline. The rate at which an individual turns gray depends on genetics. It is not uncommon to observe kinships with marked early graying. The rate of graying is also highly variable, not only on different areas of the scalp but also across the body. This may reflect variations in original melanocyte precursor seedings during melanoblast migrations in embryogenesis or in differences of niche quality. [9] Beard and body hair are affected later. Chest, pubic, and axillary hair may remain pigmented even in old age. [22] Jo et al. reported temporal and occipital areas to be more commonly involved in men than in women, with graying usually starting in the temporal area in men but in the frontal area in women. Initially involved scalp regions were also different depending on age of onset; that is, parietal or occipital area was more involved at onset in early-onset group, whereas frontal area was more involved initially in late-onset group. Early onset did not mean faster progress. Rather, the extent of grayness sharply increased after the fifth decade regardless of age at onset. [41] Graying of hair is usually progressive and permanent, but there are occasional reports of spontaneous repigmentation of gray hair, and partial, spontaneous reversal of canities may occur during the early stages of canities, whereby melanogenesis in de-activated bulbar melanocytes is re-started during anagen of the same hair growth cycle. [4]
Some authors believe that the gray color is derived from an admixture of fully white and fully pigmented hair. Canities may affect individual hair follicles during a single anagen VI growth phase, such that there is a gradual loss of pigment along the same hair shaft. [9] Full range of color from normal to white can be seen both along individual hair and from hair to hair. Finlay et al. reported that the perception of hair color is affected by the physical characteristics of the hair shaft and may bear little relationship to the true chromacities of the shaft. [50] It has been suggested that increased reflection of light may occur on cell interfaces and islets of interfibrillary matrix. [14] The white color of canities is an optical effect, that is, the reflection/refraction of incident light masks the intrinsic pale yellow color of hair keratin. [4] True gray hair are not common till old age and need to be differentiated from white hair. [51] White hair have no melanocytes or pigmentation, while gray hair has some persisting color with aberrantly distributed melanosomes. White hair usually affects only the scalp and about 5% of individuals will have whitening of hair by the fourth decade of life. [49]
Characteristics of Gray Hair
Gray hair is believed to be coarser, stiffer, and less manageable than pigmented hair. [35],[52] Gao et al. reported that gray hair undergoes more severe UV damage and needs more UV protection than dark brown hair. [53] Gray hair often fails to hold a temporary or permanent set, and is more resistant to incorporating artificial color possibly due to changes in the underlying substructure of the hair fiber. Melanin transfer possibly decreases keratinocyte turnover and increases keratinocyte terminal differentiation. Aging hair follicles may thus reprogram their matrix keratinocytes to increase production of medullary rather than cortical keratinocytes resulting in an enlarged and collapsed medulla, forming a central cavity in gray and white hair. [33] This may provide enhanced insulation to compensate for the loss of sunlight-absorbing and heat-trapping properties of pigmented dark hair. [4] White hair was also found to have increased sensitivity to weathering, increased cysteic acid residues and decreased cystine, and increased fiber reactivity to reducing and oxidizing agents. [52] Thickness, growth rate, and hair shaft elongation in non-melanized hair are significantly greater than in melanized hair. [13],[33],[34],[35] White beard hair has been shown to grow up to four times and have thicker hair shafts than pigmented beard hair. [34] Besides, lack of melanin chromophore in gray and white facial hair makes removal by laser a difficult and complicated task. [54]
Canities as a Risk Factor for Systemic Disease
Premature hair graying is considered analogous to aging and thought to reflect the aging process happening inside. Few studies showed premature hair graying occurring before the age of 40 years to be an important predictor of low bone density and osteopenia. [55],[56] The association could be part of its association with other autoimmune disorders such as vitiligo, Addison′s disease, Grave′s disease, premature hypogonadism, and Werner′s syndrome. Alternatively, premature hair graying has been shown to be less frequent in racial groups with higher bone density, suggesting a possible genetic linkage between these conditions. [55] Possibility of a common undefined causative factor accelerating both the conditions needs further exploration. Contrary to this, Morton et al. found no association between premature graying of hair and low bone mineral density. [57]
Various authors have reported premature graying of hair to be a significant risk factor for coronary artery disease (CAD). [58],[59],[60] Dwivedi et al. reported that young CAD patients who are heavy smokers also developed premature graying and balding. They suggested that presence of premature hair graying in chronic smokers indicates higher than normal risk for CAD. [60] Further, hair graying has recently been shown to be a marker of CAD independent of age and other traditional risk factors in a cohort of 213 men undergoing coronary angiography. [61] In the Copenhagen City Heart Study, Schnohr et al. reported that risk of myocardial infarction was directly proportional to the extent of graying of hair in men. [58] However, in women, the association was weaker and statistically insignificant. [58] Further, no association was evident with life longevity in the same study population. [62] Glasser et al. also found no association between premature graying of hair and increased cardiovascular morbidity, age, or cause of death. [63]
Differential Diagnosis
Canities needs to be differentiated from hypomelanotic hair disorders. The latter may present in a diffuse or localized fashion. Pigmentary dilution disorders include various types of oculocutaneous albinism including Hermansky-Pudlak and Chiedak-Higashi syndromes and Tietz syndrome. Disorders with disrupted melanosomal transfer resulting in characteristic silver hair include Griscelli, Elejalde, and Chediak-Higashi syndromes. CROSS syndrome may also present with silvery hair. In Menke′s syndrome, hair are sparse and light colored with a steel wool quality and associated with shaft abnormalities. Metabolic syndromes like phenylketonuria, histidinemia, and homocystinuria may also present with light-colored hair. Oasthouse disease, a disorder of methionine metabolism, also presents with light hair and recurrent edema. [14] Localized whitening of hair, known as poliosis, may be seen in vitiligo, piebaldism, Wardenburg syndrome, Woolf syndrome, Ziprkowski Margolis syndrome, and tuberous sclerosis. An acquired localized area of white hair should prompt the clinician to look for depigmentation of underlying skin to rule out vitiligo [Figure - 5]. Reports of sudden overnight graying of hair (canities subita) have been attributed to vitiligo, telogen effluvium, and alopecia areata. [64] An acute episode of alopecia areata may present with very sudden overnight graying due to preferential targeting of pigmented hair by the autoimmune pathology; this never occurs in true canities. [4]
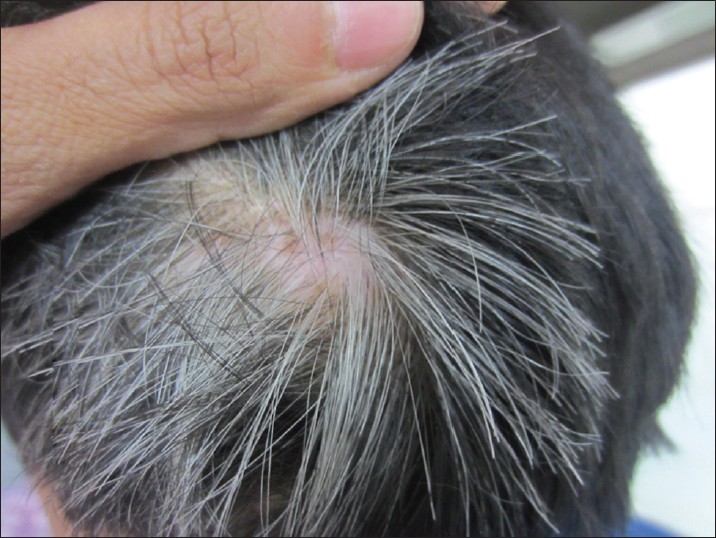 |
| Figure 5: Localized whitening of hair in a child with vitiligo (leukotrichia) |
Investigations
Diagnosis of canities is primarily clinical. Certain investigations such as serum vitamin B12, folic acid, and thyroid profile may be conducted in individuals with very early onset in the absence of any family history.
Management
Despite the extensive molecular research being carried out to understand the pathogenesis of canities, treatment options still remain far from satisfactory and no effective therapy is available. Few oral therapies have been tried with rather inconsistent results. Reports of successful treatment are anecdotal and have never been confirmed by other trials. This is surprising in view of the large number of patients presenting with premature canities to the dermatology outpatient department and the deep psychological and social impact of this sign of aging. Thus, patients are often arbitrarily prescribed nutritional supplements containing various combinations of vitamins and minerals like biotin, calcium pantothenate, zinc, copper, and selenium. However, till date, the scientific level of evidence in published literature for their efficacy is low.
Temporary hair darkening has been reported after ingestion of large doses of p-aminobenzoic acid (PABA) though the mechanism of action is unknown. [18],[65],[66] In a study comprising 460 gray-haired individuals, 100 mg three times daily of PABA caused darkening of hair in 82% patients within 2-4 months. However, relapse was evident at 2-4 weeks after drug cessation. [18],[65] Zarafonetis reported repigmentation of hair with 12-20 g of PABA. [66] Pasricha et al. reported successful use of 200 mg of calcium pantothenate daily in two girls having premature graying of hair. On a follow-up of 29 and 13 months, respectively, 300 and 1069 gray hair were counted to have got converted into black hair. Hair with a proximal black portion and a distal gray part were termed as converted hair. [67] In another study, they combined calcium pantothenate with gray hair avulsion; at every 3-monthly follow-up, all gray hair were avulsed from the root while any converted hair was snipped at the gray-black junction. They found the combination of gray hair avulsion and calcium pantothenate to be more effective than calcium pantothenate used alone. [68] Brandaleone et al., however, used 200 mg of PABA with 100 mg of calcium pantothenate (vitamin B5) and 50 g of brewer′s yeast for 8 months to patients with gray hair without any success. [69] Pavithran et al. reported PUVASOL to be effective in almost two-thirds of patients with premature graying. [70]
Repigmentation of previously gray scalp hair has been reported following prolonged (around 3 years) use of latanoprost, a PGF 2 alpha eye drops. The repigmentation started from the root and proximal portion of hair and then increased over the entire length of hair. [71] Prostaglandins are one of the most potent stimulators of melanocyte growth and melanogenesis. [72],[73] Darkening of hair has also been reported as an incidental finding with other drugs such as defibrotide, cyclosporine, corticosteroid, etretinate, L-thyroxine, verapamil, tamoxifen, levodopa, cisplatin, acitretin, tri-iodothyronine, and lenalidomide. [71],[74],[75],[76],[77] However, in most of these cases, drug-induced etiology of pigmentation could only be confirmed if hair had returned to its original color after drug withdrawal. The same could not be confirmed as most patients were continued on treatment with the suspected drug.
Repigmentation of previously gray hair has also been reported after inflammatory processes affecting the scalp, including carbuncles, erosive candidiasis of scalp, and exudative red dermatitis on sun-exposed areas. [78],[79],[80] Hair darkening has also been described after X-ray irradiation and following electron beam therapy. [51] Shaffrali et al. reported darkening of gray hair in two patients with porphyria cutanea tarda. [7] Reversal of canities in the patients mentioned above is likely to result from radiation or cytokine-induced activation of ORS melanocytes. [4]
Paucity of systemic or topical therapies in this condition has rendered camouflage techniques using hair colorants as the mainstay of therapy. The use of hair color in a patient depends on various factors such as age of onset and psychosocial impact, especially in terms of career opportunities. Based on the extent of graying, different options can be adopted. Plucking out of hair alone may be a reasonable choice if less than 10% of hair are affected. Alternatively, an individual may choose to color only the gray hair, especially in the beginning when graying is confined to the temples in men or the perimeter in women.
Major types of hair colors currently used are: temporary (textile dyes), natural coloring (e.g., henna), semi-permanent, and permanent. [65] These may also be classified as naturally occurring or synthetic. Natural dyes can be of two types: substantive and adjective. Adjective dyes alone cannot penetrate the hair sufficiently to keep from washing or fading away and require a mordant to adhere to the fiber. The mordant joins with the fiber and the dye to set the color permanently. The mordant enters deeply into the fiber, and when the dye is added, the color is formed. Substantive dyes, on the other hand, do not need mordant to adhere to the fiber. Traditionally used therapies to darken gray hair include: Amalaki (Emblica officinalis), Bhringaraj (Eclipta alba), mooncake seed tree (Sterculia platanifolia), and the lotus tree (Zizyphus spina-christi). [81] Henna, obtained from the leaves of the plant Lawsonia alba, is a naturally occurring hair colorant. It gives the auburn color and is a substantive dye for keratin in acid solutions. Henna carries the major advantage of being hypo-allergenic and non-toxic in its pure form. Although the color can add red highlights to hair, occasionally on gray hair it may come out looking orange. The chemical lawsone can also be obtained from Juglans regia or walnut. [81]
Temporary hair colorants consist of large molecules that do not penetrate the cuticle and remain adherent to the hair shaft and wash out with the next shampoo. Synthetic dyes may also be classified as: oxidative (permanent) or non-oxidative (semi-permanent) [Table - 2]. Oxidative dyes include permanent hair dyes, semi-permanent hair dyes, and auto-oxidative dyes. The most frequently used hair colorant is permanent hair dye. All permanent hair colors contain three components: an oxidation base also known as developer or primary intermediates (e.g. p-phenylenediamine, p-aminophenol, and their derivatives), a coupler (e.g. m-phenylenediamines, resorcinol, naphthols), and the oxidant (i.e. hydrogen peroxide with an alkali, usually ammonia). The couplers modify the color when used with the developer and the oxidant. The oxidant oxidizes the primary intermediates and, in combination with ammonia, lightens the natural hair color. [82] Increase in pH on addition of ammonia causes swelling of the hair fiber and opens the cuticle of the hair, allowing the color pigments to penetrate deep into the hair shaft. When the color containing alkalizing agent is combined with the developer, the peroxide becomes alkaline and diffuses through the cuticle into the hair cortex where melanin in located. The formation of colored molecules from their precursors occurs inside the hair fibers as a result of oxidation by hydrogen peroxide. Damage of the hair shaft due to oxidation reaction is the major disadvantage associated with use of permanent hair color. [82] Auto-oxidative hair dyeing involves the oxidation of dye precursors by oxygen in air without additional oxidant. Hair is not lightened and is therefore more suited for individuals with gray hair. [82]
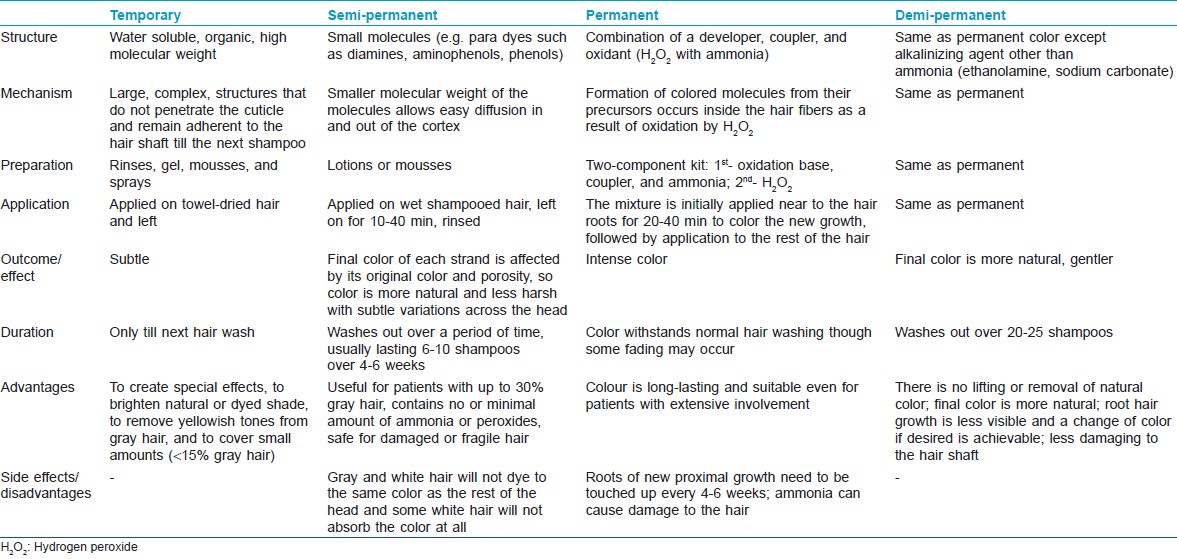
Besides concealing the undesirable gray hair, hair dyes may also protect against photodamage. Dyed hair shows a slower rate of degradation upon photo-irradiation, as compared to the undyed hair. [84] Silicone resins like trimethylsiloxysilicate and propylphenylsilsesquioxane incorporated into hair dyes have been reported to decrease the color change induced by UV radiation in dyed hair. [85]
Such products have been safely used with excellent results in millions of individuals worldwide. Studies have raised the possibility that long-term usage of permanent hair dyes (particularly black dyes) may be associated with an increased risk of developing certain cancers like lymphomas and bladder cancers. Till date, the evidence is insufficient to state with certainty whether there is a link between using hair dye and cancer. However, irritant and contact allergic reactions may develop (commonly due to p-phenylenediamine) and can result in dermatitis and sometimes hair loss. [65]
Recent advances in the management of aging hair and scalp are anti-aging compounds. Shampoos are largely ineffective as anti-aging agents due to water dilution and short contact time, and antioxidants such as vitamin C and E in these preparations protect fatty substances in the shampoo from oxidation, and not the hair. Topical anti-aging compounds of current interest are green tea polyphenols, selenium, copper, phytoestrogens, melatonin, and as yet unidentified substances from traditional Chinese medicine (TCM) and Ayurvedic medicine. Use of hormonal anti-aging protocols containing recombinant human growth hormone has resulted in improvement of hair thickness, hair growth, and in some cases darkening of hair. [65] Use of L-methionine to suppress methionine oxidation and restore the methionine-sulfoxide repair mechanism, and thus prevent graying of hair needs further exploration. [26] A new type of compounds (SkQs) comprising plastoquinone (an antioxidant moiety), a penetrating cation, and a decane or pentane linker have been synthesized that specifically target mitochondria and act as rechargeable antioxidants. These have been shown to inhibit development of age-related diseases and traits such as cataract, retinopathy, glaucoma, balding, canities, and osteoporosis in animals. [86]
Premature graying of hair is a bothersome and disfiguring condition causing significant interference with social adjustment and acceptance, hence the need for identifying effective and long-lasting treatment options. The future of treatment options for premature canities lies with targeting genes and proteins involved in hair follicle melanocyte biology. These may aid in developing natural, biotechnological, or semi-synthetic hair repigmentation techniques including newer methods for delivering coloring agents directly to the hair follicle. Further research into the pathophysiology of hair graying not only will reveal promising targets for intervention, but may also provide useful links to the understanding of the aging process as a whole.
| 1. |
Wood JM, Jimbow K, Boissy RE, Slominski A, Plonka PM, Slawinski J, et al. What's the use of generating melanin? Exp Dermatol 1999;8:153-64.
[Google Scholar]
|
| 2. |
Rees J. Plenty new under the sun. J Invest Dermatol 2006;126:1691-2.
[Google Scholar]
|
| 3. |
Rees JL. The melanocortin 1 receptor (MC1R): More than just red hair. Pigment Cell Res 2000;13:135-40.
[Google Scholar]
|
| 4. |
Tobin DJ, Paus R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp Gerontol 2001;36:29-54.
[Google Scholar]
|
| 5. |
Tobin DJ, Bystryn JC. Different populations of melanocytes are present in hair follicles and epidermis. Pigment Cell Res 1996;9:304-10.
[Google Scholar]
|
| 6. |
Horikawa T, Norris DA, Johnson TW, Zekman T, Dunscomb N, Bennion SD, et al. DOPA-negative melanocytes in the outer root sheath of human hair follicles express premelanosomal antigens but not a melanosomal antigen or the melanosome-associated glycoproteins tyrosinase, TRP-1, and TRP-2. J Invest Dermatol 1996;106:28-35.
[Google Scholar]
|
| 7. |
Shaffrali FC, McDonagh AJ, Messenger AG. Hair darkening in porphyria cutanea tarda. Br J Dermatol 2002;146:325-9.
[Google Scholar]
|
| 8. |
Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev 2001;81:449-94.
[Google Scholar]
|
| 9. |
Tobin DJ. Aging of the hair follicle pigmentation system. Int J Trichology. 2009;1:83-93.
[Google Scholar]
|
| 10. |
Sunderland E. Hair-colour variation in the United Kingdom. Ann Hum Genet 1956;20:312-33.
[Google Scholar]
|
| 11. |
Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: Incomplete melanocyte stem cell maintenance in the niche. Science 2005;307:720-4.
[Google Scholar]
|
| 12. |
Peters EM, Imfeld D, Gräub R. Graying of the human hair follicle. J Cosmet Sci 2011;62:121-5.
[Google Scholar]
|
| 13. |
Arck PC, Overall R, Spatz K, Liezman C, Handjiski B, Klapp BF, et al. Towards a "free radical theory of graying": Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J 2006;20:1567-9.
[Google Scholar]
|
| 14. |
Dawber RP, Gummer CL. The colour of the hair. In: Dawber R editor. Diseases of the hair and scalp. 3 rd ed. Oxford: Blackwell Science; 1997.p. 397-416.
[Google Scholar]
|
| 15. |
Lorincz AL. Disturbances of Melanin Pigmentation. In: Moschella SL, Hurley HJ editors. Dermatology Moschella and Hurley, 2 nd ed. Philadelphia: WS Saunders; 1985.p. 1290-317.
[Google Scholar]
|
| 16. |
Dawber RP. Integumentary associations of pernicious anaemia.Br J Dermatol 1970;82:221-3.
[Google Scholar]
|
| 17. |
Fatemi Naieni F, Ebrahimi B, Vakilian HR, Shahmoradi Z. Serum iron, zinc, and copper concentration in premature graying of hair. Biol Trace Elem Res 2012;146:30-4.
[Google Scholar]
|
| 18. |
Wadhwa SL, Khopkar U, Nischal KC. Hair and scalp disorders. In: Valia RG, Valia AR editors. IADVL Textbook of Dermatology. 3 rd ed. Mumbai: Bhalani Publishing House; 2008.p. 864-948.
[Google Scholar]
|
| 19. |
Balagula Y, Pulitzer MP, Maki RG, Myskowski PL. Pigmentary changes in a patient treated with imatinib. J Drugs Dermatol 2011;10:1062-6.
[Google Scholar]
|
| 20. |
Dalgic B, Egritas O. Gray hair and acrodermatitis enteropathica-like dermatitis: An unexpected presentation of cystic fibrosis. Eur J Pediatr 2011;170:1305-8.
[Google Scholar]
|
| 21. |
Trakymiene SS, Abla O. Hodgkin lymphoma presenting with hair graying. J Pediatr Hematol Oncol 2010;32:417-8.
[Google Scholar]
|
| 22. |
Mosher DB, Fitzpatrick TB, Ortonne JP, Hori Y. Hypomelanoses and hypermelanosis. Disorders of melanocytes. In: Freedmerg IM, Eisen AZ, Katz SI, Wolff K, Goldsmith LA, Austen KF, et al. editors. Fitzpatrick's Dermatology in General Medicine. 5 th ed. New York: McGraw Hill; 1999.p. 945-1017.
th ed. New York: McGraw Hill; 1999.p. 945-1017.'>[Google Scholar]
|
| 23. |
Fitch N, Pinsky L, Lachance RC. A form of bird-headed dwarfism with features of premature senility. Am J Dis Child 1970;120:260-4.
[Google Scholar]
|
| 24. |
Daneshpazhooh M, Nazemi TM, Bigdeloo L, Yoosefi M. Mucocutaneous findings in 100 children with Down syndrome. Pediatr Dermatol 2007;24:317-20.
[Google Scholar]
|
| 25. |
Trüeb RM. Oxidative stress in ageing of hair. Int J Trichol 2009;1:6-14.
[Google Scholar]
|
| 26. |
Wood JM, Decker H, Hartmann H, Chavan B, Rokos H, Spencer JD, et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J 2009;23:2065-75.
[Google Scholar]
|
| 27. |
Kadekaro AL, Kavanagh RJ, Wakamatsu K, Ito S, Pipitone MA, Abdel-Malek ZA. Cutaneous photobiology. The melanocyte vs. the sun: Who will win the final round? Pigment Cell Res 2003;16:434-47.
[Google Scholar]
|
| 28. |
Emerit I, Filipe P, Freitas J, Vassy J. Protective effect of superoxide dismutase against hair graying in a mouse model. Photochem Photobiol 2004;80:579-82.
[Google Scholar]
|
| 29. |
Irie M, Asami S, Nagata S, Miyata M, Kasai H. Relationships between perceived workload, stress and oxidative DNA damage. Int Arch Occup Environ Health 2001:74:153-7.
[Google Scholar]
|
| 30. |
Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Nat Acad Sci U S A 2004;101:17312-5.
[Google Scholar]
|
| 31. |
Fuchs J, Zollner TM, Kaufmann R, Podda M. Redox-modulated pathways in inflammatory skin diseases. Free Radic Biol Med 2001;30:337-53.
[Google Scholar]
|
| 32. |
Akar A, Arca E, Erbil H, Akay C, Sayal A, Gur AR. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J Dermatol Sci 2002;29:85-90.
[Google Scholar]
|
| 33. |
Van Neste D. Thickness, medullation and growth rate of female scalp hair are subject to significant variation according to pigmentation and scalp location during ageing. Eur J Dermatol 2004;14:28-32.
[Google Scholar]
|
| 34. |
Nagl W. Different growth rates of pigmented and white hair in the beard: Differentiation vs. proliferation? Br J Dermatol 1995;132:94-7.
[Google Scholar]
|
| 35. |
Choi HI, Choi GI, Kim EK, Choi YJ, Sohn KC, Lee Y, et al. Hair greying is associated with active hair growth. Br J Dermatol 2011;165:1183-9.
[Google Scholar]
|
| 36. |
Paus R. A neuroendocrinological perspective on human hair follicle pigmentation. Pigment Cell Melanoma Res 2011;24:89-106.
[Google Scholar]
|
| 37. |
Meyer KC, Brzoska T, Abels C, Paus R. The alpha-melanocyte stimulating hormone-related tripeptide K(D) PT stimulates human hair follicle pigmentation in situ under proinflammatory conditions. Br J Dermatol 2009;160:433-7.
[Google Scholar]
|
| 38. |
Westerhof W, Njoo D, Menke KE. Miscellaneous hypomelanoses: Disorders characterized by extracutaneous loss of pigmentation. In: Nordlund JJ, Biossy RE, Hearing VJ, King RA, Ortonne JP. editors. The Pigmentary System: Physiology and Pathophysiology. New York: Oxford University Press; 1998.p. 690-705.
[Google Scholar]
|
| 39. |
Ortonne JP, Thivolet J, Guillet R. Graying of hair with age and sympathectomy. Arch Dermatol 1982;118:876-7.
[Google Scholar]
|
| 40. |
Lemer AB. Gray hair and sympathectomy. Report of a case. Arch Dermatol 1966;93:235-6.
[Google Scholar]
|
| 41. |
Jo SJ, Paik SH, Choi JW, Lee JH, Cho S, Kim KH, et al. Hair graying pattern depends on gender, onset age and smoking habits. Acta Derm Venereol 2012;92:160-1.
[Google Scholar]
|
| 42. |
Reece AS. Hair graying in substance addiction. Arch Dermatol 2007;143:116-8.
[Google Scholar]
|
| 43. |
Thomas J, Prabhavathy D, Augustine SM, Muthuswami TC. Absence of graying of pinnal hairs. Arch Dermatol 1989;125:1589.
[Google Scholar]
|
| 44. |
Han R, Beppu H, Lee YK, Georgopoulos K, Larue L, Li E, et al. A pair of transmembrane receptors essential for the retention and pigmentation of hair. Genesis 2012;50:783-800.
[Google Scholar]
|
| 45. |
Hachiya A, Sriwiriyanont P, Kobayashi T, Nagasawa A, Yoshida H, Ohuchi A, et al. Stem cell factor-KIT signalling plays a pivotal role in regulating pigmentation in mammalian hair. J Pathol 2009;218:30-9.
[Google Scholar]
|
| 46. |
Schouwey K, Delmas V, Larue L, Zimber-Strobl U, Strobl LJ, Radtke F, et al. Notch 1 and Notch 2 receptors influence progressive hair graying in a dose-dependent manner. Dev Dyn 2007;236:282-9.
[Google Scholar]
|
| 47. |
McConnell AA, Wade WG, Milliken TG. A study of two families with multiple autoimmune disease. Ir J Med Sci 1970;3:463-73.
[Google Scholar]
|
| 48. |
Panhard S, Lozano I, Loussouarn G. Greying of the human hair. A worldwide survey, re-visiting the "50" rule of thumb. Br J Dermatol 2012.
[Google Scholar]
|
| 49. |
Keogh EV, Walsh RJ. Rate of greying of human hair. Nature 1965;207:877-8.
[Google Scholar]
|
| 50. |
Findlay GH. An optical study of human hair colour in normal and abnormal conditions.Br J Dermatol 1982;107:517-27.
[Google Scholar]
|
| 51. |
Messenger AG. The control of hair growth and pigmentation. In: Olsen EA. editor. Disorders of Hair Growth: Diagnosis and Treatment. New York: McGraw-Hill; 1994.p. 39-58.
[Google Scholar]
|
| 52. |
Hollfelder B, Blankenburg G, Wolfram LJ, Hocker Hl. Chemical and physical properties of pigmented and non-pigmented hair ('grey hair'). Int J Cosmet Sci 1995;17:87-9.
[Google Scholar]
|
| 53. |
Gao T, Bedell A. Ultraviolet damage on natural gray hair and its photoprotection. J Cosmet Sci 2001;52:103-18.
[Google Scholar]
|
| 54. |
Alijanpoor R, Bejehmir AP, Mokmeli S. Successful white hair removal with combined coloring and intense pulsed Light (IPL): A randomized clinical trial. Photomed Laser Surg 2011;29:773-9.
[Google Scholar]
|
| 55. |
Orr-Walker BJ, Evans MC, Ames RW, Clearwater JM, Reid IR. Premature hair graying and bone mineral density. J Clin Endocrinol Metab 1997;82:3580-3.
[Google Scholar]
|
| 56. |
Rosen CJ, Holick MF, Millard PS. Premature graying of hair is a risk marker for osteopenia. J Clin Endocrinol Metab 1994;79:854-7.
[Google Scholar]
|
| 57. |
Morton DJ, Kritz-Silverstein D, Riley DJ, Barrett-Connor EL, Wingard DL. Premature graying, balding, and low bone mineral density in older women and men: The Rancho Bernardo study. J Aging Health 2007;19:275-85.
[Google Scholar]
|
| 58. |
Schnohr P, Lange P, Nyboe J, Appleyard M, Jensen G. Gray hair, baldness, and wrinkles in relation to myocardial infarction: The Copenhagen City Heart Study. Am Heart J 1995;130:1003-10.
[Google Scholar]
|
| 59. |
Gould L, Reddy CV, Oh KC, Kim SG, Becker W. Premature hair graying: A probable coronary risk factor. Angiology 1978;29:800-3.
[Google Scholar]
|
| 60. |
Dwivedi S, Jhamb R. Cutaneous markers of coronary artery disease. World J Cardiol 2010;2:262-9.
[Google Scholar]
|
| 61. |
Kocaman SA, Cetin M, Durakoðlugil ME, Erdoðan T, Canga A, Ciçek Y, et al . The degree of premature hair graying as an independent risk marker for coronary artery disease: A predictor of biological age rather than chronological age. Anadolu Kardiyol Derg 2012;12:457-63.
[Google Scholar]
|
| 62. |
Schnohr P, Nyboe J, Lange P, Jensen G. Longevity and gray hair, baldness, facial wrinkles, and arcus senilis in 13,000 men and women: The Copenhagen City Heart Study. J Gerontol A Biol Sci Med Sci 1998;53:M347-50.
[Google Scholar]
|
| 63. |
Glasser M. Is early onset of gray hair a risk factor? Med Hypotheses 1991;36:404-11.
[Google Scholar]
|
| 64. |
Tan SP, Weller RB. Sudden whitening of the hair in an 82-year-old woman: The'overnight greying' phenomenon. Clin Exp Dermatol 2012;37:458-9.
[Google Scholar]
|
| 65. |
Trüeb RM. Pharmacologic interventions in aging hair. Clin Interv Aging 2006;1:121-9.
[Google Scholar]
|
| 66. |
Zarafonetis C. Darkening of gray hair during para-aminobenzoic acid therapy. J Invest Dermatol 1950;15:399-401.
[Google Scholar]
|
| 67. |
Pasricha JS. Successful treatment of gray hairs with high dose calcium pantothenate. Indian J Dermatol Venereol Leprol 1981:47:311-3.
[Google Scholar]
|
| 68. |
Pasricha JS. Effect of grey hair avulsion on the response to calcium pantothente in premature grey hairs. Indian J Dermatol Venereol Leprol 1986:52:77-80.
[Google Scholar]
|
| 69. |
Brandaleone H, Main E, Steele JM. The effect of calcium pantothenate and para-aminobenzoic acid on gray hair in man.A Study on a group of younger and older individuals. Am J Med Sci 1944;208:315-20.
[Google Scholar]
|
| 70. |
Pavithran K. PUVASOL therapy in premature graying. Indian J Dermatol Venereol Leprol 1986;52:54-8.
[Google Scholar]
|
| 71. |
Bellandi S, Amato L, Cipollini EM, Antiga E, Brandini L, Fabbri P. Repigmentation of hair after latanoprost therapy. J Eur Acad Dermatol Venereol 2011;25:1485-7.
[Google Scholar]
|
| 72. |
Abdel-Malek ZA, Swope VB, Amornsiripanitch N, Nordlund JJ. In vitro modulation of proliferation and melanization of S91 melanoma cells by prostaglandins. Cancer Res 1987;47:3141-6.
[Google Scholar]
|
| 73. |
Nordlund JJ, Collins CE, Rheins LA. Prostaglandin E2 and D2 but not MSH stimulate the proliferation of pigment cells in the pinnal epidermis of the DBA / 2 mouse. J Invest Dermatol 1986;86:433-7.
[Google Scholar]
|
| 74. |
Rubegni P, Sbano P, Fimiani M. A case of disseminated granuloma annulare treated with defibrotide: Complete clinical remission and progressive hair darkening. Br J Dermatol 2003;149:437-9.
[Google Scholar]
|
| 75. |
Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol 2009;50:214-6.
[Google Scholar]
|
| 76. |
Sadighha A, Zahed GM. Hair darkening after treatment with cyclosporin in a patient with psoriasis. J Eur Acad Dermatol Venereol 2008;22:1239-41.
[Google Scholar]
|
| 77. |
Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: Graying may not be an irreversible process. J Oncol Pharm Pract 2013;19:165-9.
[Google Scholar]
|
| 78. |
Pinkus H. Postinflammatory hair darkening. Arch Dermatol 1960;82:263-4.
[Google Scholar]
|
| 79. |
Verbov J. Erosive candidiasis of the scalp, followed by the reappearance of black hair after 40 years. Br J Dermatol 1981;105:595-8.
[Google Scholar]
|
| 80. |
Bhutani LK, Minocha YK, Dhir GC, Rao DS. Repigmentation of hair following photosensitive dermatitis. Dermatologica 1978;156:101-4.
[Google Scholar]
|
| 81. |
Dweck AC. Natural ingredients for colouring and styling. Int J Cosmet Sci 2002;24:287-302.
[Google Scholar]
|
| 82. |
Morel OJ, Christie RM. Current trends in the chemistry of permanent hair dyeing. Chem Rev 2011;111:2537-61.
[Google Scholar]
|
| 83. |
Bolduc C, Shapiro J. Hair Care Products: Waving, Straightening, onditioning, and Coloring. Clin Dermatol 2001;19:431-6.
[Google Scholar]
|
| 84. |
Pande CM, Albrecht L, Yang B. Hair photoprotection by dyes. J Cosmet Sci 2001;52:377-89.
[Google Scholar]
|
| 85. |
Schlosser A. Silicones used in permanent and semi-permanent hair dyes to reduce the fading and color change process of dyed hair occurred by wash-out or UV radiation. J Cosmet Sci 2004;55 Suppl: S123-31.
[Google Scholar]
|
| 86. |
Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, et al. An attempt to prevent senescence: A mitochondrial approach. Biochim Biophys Acta 2009;1787:437-61.
[Google Scholar]
|
Fulltext Views
76,460
PDF downloads
10,028





