Translate this page into:
Prevalence of ocular findings in a sample of Egyptian patients with psoriasis
2 Department of Ophthalmology, Faculty of Medicine, Alexandria University, Alexandria, Egypt
Correspondence Address:
Salma Samir Omar
Elkhartoum Square, Alexandria 21521
Egypt
| How to cite this article: Omar SS, Helaly HA. Prevalence of ocular findings in a sample of Egyptian patients with psoriasis. Indian J Dermatol Venereol Leprol 2018;84:34-38 |
Abstract
Background: Psoriasis is a common disorder worldwide. The prevalence of psoriasis in Egypt, an African country with a Caucasian population, ranges 0.19–3%. Despite this relatively high prevalence of psoriasis, there are no epidemiologic data regarding the burden of associated eye affection. Determining the magnitude of the problem could help in offering better integrated health services.Aim: The purpose of this study was to evaluate eye involvement in a sample of Egyptian psoriatic patients.
Patients and Methods: This case-control study included 100 patients with psoriasis and 100 age and sex matched healthy controls. Psoriasis extent and severity was graded by psoriasis area and severity index (PASI). Complete ophthalmological examination and tests for dry eye were performed to all subjects.
Results: The mean age of the psoriasis group was 50.7 ± 14.3 years. Thirty eight percent of the cases were females. The mean duration of psoriasis was 10.1 ± 7.5 years. Psoriasis patients had more conjunctival injection (n = 40, P = 0.035), more pinguecula (n = 38, P = 0.048) than controls. Ocular surface disease index (OSDI), tear breakup time (TBUT), Schirmer I, and Rose Bengal staining showed statistically significant positive results in the psoriasis group.
Conclusion: This is the first report on the prevalence of eye comorbidities in Egyptian psoriatic patients. Dry eyes were more common with psoriasis, particularly the erythrodermic type. Other ocular findings were not statistically significantly different except for conjunctival injection and pinguecula.
Introduction
Psoriasis is a common inflammatory disorder affecting both sexes, all age groups, and skin types.[1] The global incidence is reported to be on the rise.[2] It affects about 3–4% of people worldwide.[3] This global prevalence of psoriasis is variable in different countries and regions all over the world.[2] According to a 2013 systematic review of published population-based studies, it was found that psoriasis was more frequent in Caucasian populations compared to non-Caucasians.[4] Furthermore, East African countries have higher reported prevalence of the disease compared to West African countries.[1] The reported prevalence in Egypt varies from 0.19% to 3%.[3] However, the real disease burden is suspected to be even larger as most epidemiologic studies were based on self-reporting.[3]
Psoriasis was known to classically affect the skin, nails, and joints. However, it is now clear that psoriasis is a multisystem chronic inflammatory disorder with associated comorbidities.[5],[6],[7],[8] This emphasizes the need for a multidisciplinary approach to manage these patients and control their comorbidities.[9]
An ocular inflammatory reaction may be found associated with psoriasis. These disorders include abnormalities of the conjunctiva, cornea, sclera, uvea, and lens.[10],[11],[12] Early diagnosis and management of ocular affection would avoid serious complications and optimize patients' treatment outcome. Despite the relatively high prevalence of psoriasis in Egypt, there are no data in literature regarding the magnitude of ocular affection in Egyptian patients.
Aim
The aim of the current study was to evaluate eye involvement in a sample of Egyptian patients with psoriasis.
Patients and Methods
This case control study included 100 patients with psoriasis and 100 age and sex-matched healthy controls with no psoriasis attending the outpatient clinic at the Faculty of Medicine, Alexandria University, Egypt. All included subjects signed an informed consent. This study was approved by the local research committee of Faculty of Medicine, Alexandria University, Egypt. The tenets of Declaration of Helsinki were followed. Patients were excluded from the study if they had any inflammatory connective tissue diseases, diabetes, nephropathy, lung and heart disease, gastroenterological disease/inflammatory bowel disease, neurological disease, neoplasia, metabolic bone disease, skin diseases other than psoriasis, infections, hematological disease, liver disease, previous ocular surgery, active eye infection, or active ocular allergy.
A thorough personal, family, and drug history was taken from all subjects. The disease duration, psoriasis treatment and joint involvement were recorded. Full dermatological examination was performed by the same dermatologist. Psoriasis extent and severity was graded by psoriasis area and severity index (PASI).[13] Finger and toenails were examined for presence of psoriatic affection and the severity of nail affection, when present, was assessed by calculating the nail psoriasis severity index (NAPSI) score.[14]
Complete ophthalmological examination was performed for all subjects by the same ophthalmologist. Thepatients were not allowed to put eye drops on the day of the examination. The examination included best corrected visual acuity (BCVA), slit lamp examination for the cornea and the anterior segment of the eye to assess any inflammations, cataract, or any other pathology, intraocular pressure measurement, and posterior segment examination to evaluate the presence of any inflammatory or vascular complications.
Tests for detecting dry eye were performed. This included tear breakup time (TBUT), Schirmer I, and Rose Bengal staining tests.[15],[16],[17],[18] For the TBUT, topical fluorescein 1%was applied to the eye, and then the mean of three consecutive breakup times was calculated. A time of less than 10 s was considered abnormal. For the Schirmer I test, a 5 × 35 mm paper strip without anesthesia was applied to the lower temporal lid margin, and then the amount of paper wetting was measured. A valueofless than10 mm in 5 min was consideredabnormal.[15],[16],[17] For Rose Bengal staining test, a Rose Bengal strip was applied to the inferior bulbar conjunctiva to assess staining. The reaction was classified according to the van Bijsterveld scoring system and values of more than 3 were considered to be abnormal.[18] Theocular surface disease index (OSDI) questionnaire was used to evaluate the dry eye symptoms.[19]
Clinical findings were statistically evaluated using Excel 2007 (Microsoft Corp.) and SPSS software version 15.0 (SPSS Inc, Chicago, IL). Means and standard deviations were calculated. To check for normal distribution, the Kolmogorov-Smirnov test was applied. Comparisons of the means of normally distributed data were performed with the t-tests. Chi square and Fisher's exact tests were used to compare between different percentages. Pearson correlation was used to correlate between different variables. A P value less than 0.05 was considered statistically significant.
Results
The mean age of the psoriasis group (n = 100) was 50.7 ± 14.3 years (ranged from 21 years to 80 years). Thirty eight percent of the cases were females (n = 38). A control group was recruited and matched for the age and sex of the psoriasis group with a mean age of 51.1 ± 14.2 years and 38 females. The mean duration of psoriasis disease was 10.1 ± 7.5 years (ranged from 1 years to 32 years). The clinical type of psoriasis was chronic plaque in 82 patients (82%), erythrodermic in 13 patients, chronic generalized in three patients, and two cases were generalized pustular type. Seventeen patients (17%) received no treatment and 83 (83%) patients received treatment. The treatment was methotrexate in 65 patients, narrow band (NB) ultraviolet B (UVB) phototherapy in 16 patients, and two cases received tumor necrosis factor (TNF)-alpha receptor antagonist (anti-TNF).
The mean PASI score for the psoriasis patients was 11.7 ± 9.7 (ranged from 2 to 47). Twenty one patients (21%) suffered from arthritis. Seventy five patients (75%) had nail affection. The mean NAPSI score for the psoriasis patients was 22.8 ± 22.0 (ranged from 0 to 72). There was no significant correlation between PASI and NAPSI score among the included patients (P = 0.545). Thirty-two patients (32%) had facial involvement. One-third of the patients who had facial involvement also suffered from lid affection (n = 11).
Most of the patients of the psoriasis and control groups had BCVA of 0.3 log MAR (log of the Minimum Angle of Resolution) or better (88% of the psoriasis group and 90% of the control group). This was not a statistically significant difference (P = 0.651). The patients with worse vision were mainly due to senile cataract. Two cases (one in the psoriasis group and one in the control group) had age-related macular degeneration which caused the decrease in visual acuity.
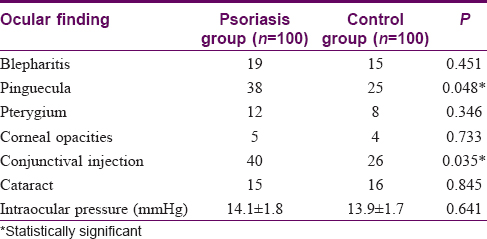
[Table - 1] shows the different ocular findings found in both psoriasis and control groups. As regards blepharitis, pterygium, and cataract, there was no statistically significant difference between the two groups. Corneal involvement ranged from mild punctate keratitis to visually significant opacities. This was not statistically significant between the two groups. Psoriasis patients had more conjunctival injection (n = 40) than healthy controls. This was statistically significant (P = 0.035). Also, psoriasis patients had statistically significant more pinguecula than healthy controls (n = 38, P = 0.048). As regards uveitis, none of the healthy controls had uveitis. Three psoriasis patients had anterior uveitis, but this number is small to be of statistical significance. All the three patients with uveitis suffered also from arthritis.
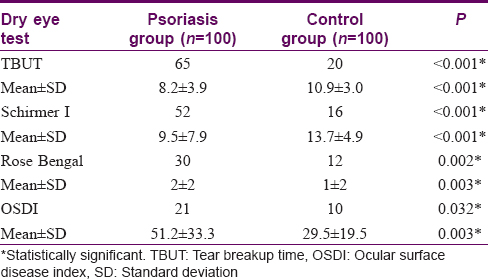
[Table - 2] shows the mean scores for dry eye tests and the number of persons with abnormal results in both psoriasis and control groups. OSDI, TBUT, Schirmer I, and Rose Bengal staining showed statistically significant positive results for dry eye in the psoriasis group in comparison with the control group. Abnormal results were obtained in 65, 52, 30, and 21 psoriasis patients as regards TBUT, Schirmer I, Rose Bengal staining, and OSDI respectively. The duration of psoriasis disease did not correlate significantly with TBUT, Schirmer I, Rose Bengal staining, and OSDI (P > 0.05). PASI score showed moderate correlation with TBUT (r = 0.497, P = 0.007), Schirmer I (r = 0.552, P = 0.002), Rose Bengal staining (r = 0.548, P = 0.003), OSDI (r = 0.444, P = 0.018) [Figure - 1], [Figure - 2], [Figure - 3].
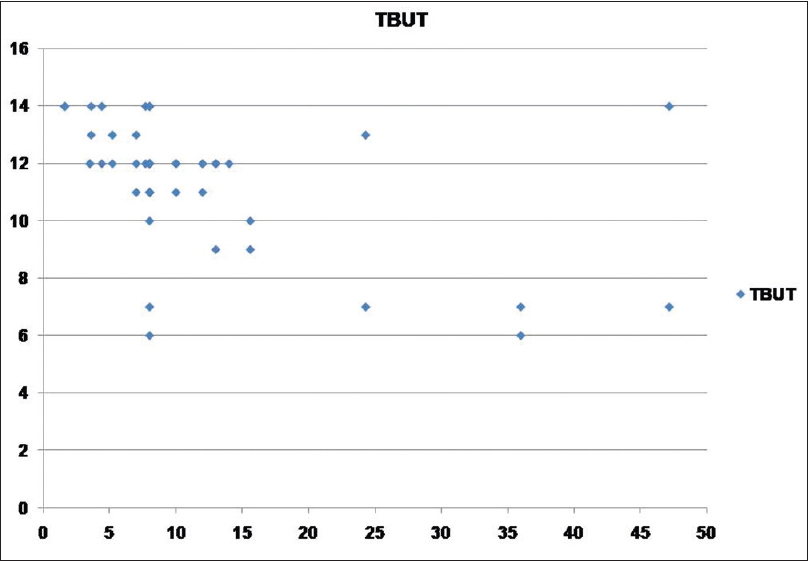 |
| Figure 1: Scatter showing relation between psoriasis area and severity index score and tear breakup time test |
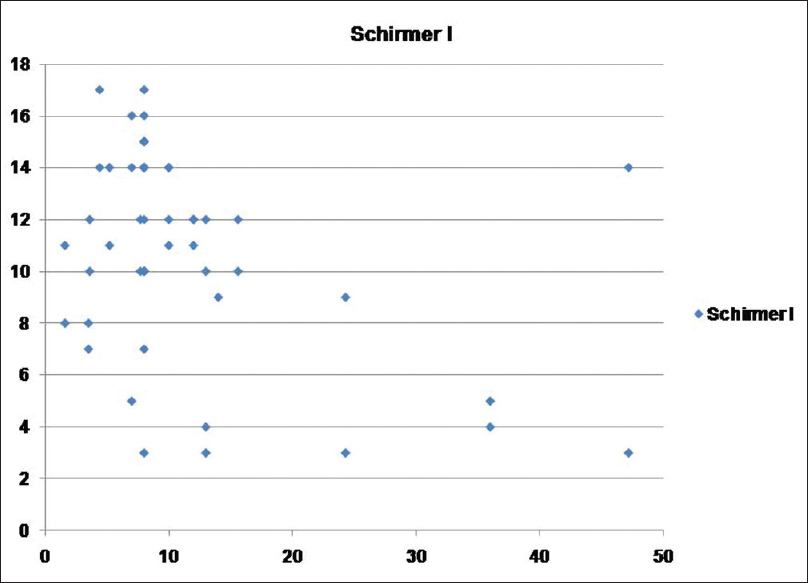 |
| Figure 2: Scatter showing relation between psoriasis area and severity index score and Schirmer I test |
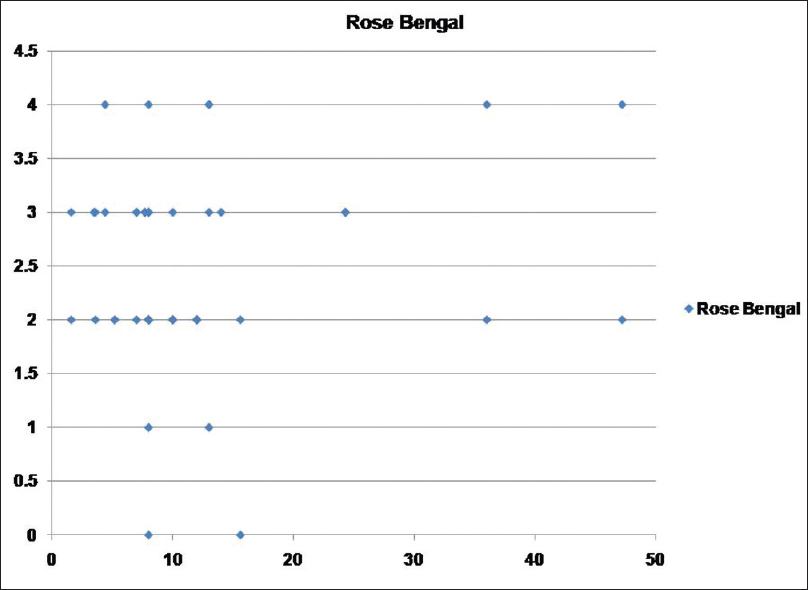 |
| Figure 3: Scatter showing relation between psoriasis area and severity index score and Rose Bengal staining score |
Subgroup analysis of psoriasis patients with facial affection or arthritis did not show any different results. Except for that all three cases with uveitis had arthritis. Subgroup analysis of patients with nail affection (n = 75) showed that they have slightly worse results as regards scores of dry eye, but this difference was not statistically significant (P > 0.05). NAPSI score failed to show statistically significant correlation with TBUT, Schirmer I, Rose Bengal staining, or OSDI. Subgroup analysis of the psoriasis patients with erythrodermic type (n = 13) showed that they had more conjunctival injection (n = 10). This was statistically significant (P = 0.0036). Also, TBUT, Schirmer I, Rose Bengal staining, and OSDI showed worse results than the other types of psoriasis. This was statistically significant (P< 0.05).
Discussion
In the current study, the authors screened 100 Egyptian patients of psoriasis for associated ocular involvement which might or might not be related to psoriasis disease or the systemic treatment. There are few reports about this subject in the literature. For the sake of comparison, a control group of healthy adults, who were matched for age and sex, were recruited. Twenty one patients (21%) suffered from arthritis. This is comparable to the reported incidence in the literature which states that it may occur in up to 30% of psoriasis patients and it is diagnosed based on clinical and radiological features.[20],[21] Two thirds of the psoriasis patients received methotrexate which is reported to have major systemic side effects like hepatic involvement.[22] None of the included patients in the current study had any serious systemic side effect.
There was no statistically significant difference as regards the BCVA of both groups. All the cataract cases were of the senile type. There was no complicated cataract. Psoriasis patients showed statistically and clinically significant more conjunctival injection and pinguecula. This might be explained by the fact that they had more dry eye symptoms and signs. As regards, the pterygium, blepharitis, and intraocular pressure, there were no statistically significant difference between the two groups. This is comparable to the results mentioned by Lima et al.[23] as regards the prevalence of eye disease in Brazilian patients with psoriatic arthritis. In the current study, the three cases with anterior uveitis had arthritis. The number of cases is too little to be statistically significant, but cases with arthritis should be monitored for uveitis due to its clinical relevance.[23],[24],[25],[11]
All the test scores for dry eye were significantly positive in the psoriasis group in comparison with the control group. The PASI score which indicated the psoriasis severity correlated significantly with the dry eye tests scores. Furthermore, subgroup analysis of the psoriasis patients with erythrodermic type (n = 13) showed that they had more conjunctival injection and more dry eye signs and symptoms. This correlation probably reflects the role of the level of systemic inflammation in psoriatic patients in the development of eye complications, and suggests that the newer biologic anti-TNF-α drugs could be a promising therapy for treating not only psoriatic lesions, but for psoriasis associated comorbidities. We could not study the statistical relation of pustular psoriasis; however, with eye findings due to the insufficient number of cases within this subgroup.
Nail psoriasis which was a common finding in the current study group did not correlate with PASI score. This agrees with the fact that nail affection can occur in 40% of patients with mild psoriasis and even in patients with no skin psoriasis.[26] We could not demonstrate a significant relationship between the involvement of nails and ocular findings. This lack of association was also shown by Kilic et al.[10] However, the worse scores on dry eye tests observed in our patients with nail psoriasis suggest that a larger number of patients may be needed to adequately test if a significant relation exists.
The number of abnormal tests was significantly higher among psoriasis patients. This number was highest with TBUT (n = 65) and least with OSDI (n = 21). This is explained by the poor relation between the signs and symptoms of the dry eye. Nichols et al.[27] also showed lack of association between signs and symptoms in patients with dry eye disease. The above mentioned statistically and clinically significant results suggest a relation between psoriasis and dry eye. Dry eye syndrome is well recognized in other systemic diseases especially rheumatologic diseases.[28],[29],[30] Prescribing artificial tear drops is important in managing those patients with dry eye to avoid complications of dry eye disease.
Conclusion
There are no available epidemiologic data regarding the magnitude of eye affection in psoriatic patients in Egypt. Our report is the first report on prevalence of eye disease in psoriasis in the Caucasian Egyptian population. Dry eyes were commoner in psoriasis patients. Other ocular findings were not statistically significantly different except for conjunctival injection and pinguecula which may also be explained by the presence of dry eye. The findings of this study were limited by small population size and inability to compare different clinical types of psoriasis as pustular psoriasis. However, it suggests that eye complications are common in our patients particularly the erythrodermic type and therefore should be addressed during history taking and an annual ophthalmologic evaluation for our psoriatic patients should be encouraged.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Alexis AF, Blackcloud P. Psoriasis in skin of color: Epidemiology, genetics, clinical presentation, and treatment nuances. J ClinAesthetDermatol 2014;7:16-24.
[Google Scholar]
|
| 2. |
Fernandez-Obregon A. Psoriasis. In: O'Daly J, editor. Psoriasis – A Systemic Disease. 1st ed. Croatia: InTech; 2012. p. 159-217.
[Google Scholar]
|
| 3. |
Cimmino MA. Epidemiology of psoriasis and psoriatic arthritis. Reumatismo 2007;59 Suppl 1:19-24.
[Google Scholar]
|
| 4. |
Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377-85.
[Google Scholar]
|
| 5. |
Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: A systematic review. Sleep Med Rev 2016;29:63-75.
[Google Scholar]
|
| 6. |
Davidovici BB, Sattar N, Prinz J, Puig L, Emery P, Barker JN, et al. Psoriasis and systemic inflammatory diseases: Potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol 2010;130:1785-96.
[Google Scholar]
|
| 7. |
Duffin KC. Identifying and managing complications and comorbidities in patients with psoriasis. SeminCutan Med Surg 2015;34 2 Suppl:S30-3.
[Google Scholar]
|
| 8. |
Husni ME. Comorbidities in psoriatic arthritis. Rheum Dis Clin North Am 2015;41:677-98.
[Google Scholar]
|
| 9. |
Gerdes S, Mrowietz U. Comorbidities and psoriasis. Impact on clinical practice. Hautarzt 2012;63:202-13.
[Google Scholar]
|
| 10. |
Kilic B, Dogan U, Parlak AH, Goksugur N, Polat M, Serin D, et al. Ocular findings in patients with psoriasis. Int J Dermatol 2013;52:554-9.
[Google Scholar]
|
| 11. |
Lambert JR, Wright V. Eye inflammation in psoriatic arthritis. Ann Rheum Dis 1976;35:354-6.
[Google Scholar]
|
| 12. |
Campanati A, Neri P, Giuliodori K, Arapi I, Carbonari G, Borioni E, et al. Psoriasis beyond the skin surface: A pilot study on the ocular involvement. IntOphthalmol 2015;35:331-40.
[Google Scholar]
|
| 13. |
Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician's global assessment. J Am AcadDermatol 2004;51:563-9.
[Google Scholar]
|
| 14. |
Mukai MM, Poffo IF, Werner B, Brenner FM, Lima Filho JH. NAPSI utilization as an evaluation method of nail psoriasis in patients using acitretin. An Bras Dermatol 2012;87:256-62.
[Google Scholar]
|
| 15. |
Shapiro A, Merin S. Schirmer test and break-up time of tear film in normal subjects. Am J Ophthalmol 1979;88:752-7.
[Google Scholar]
|
| 16. |
Lee SH, Tseng SC. Rose Bengal staining and cytologic characteristics associated with lipid tear deficiency. Am J Ophthalmol 1997;124:736-50.
[Google Scholar]
|
| 17. |
Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci 2010;51:6125-30.
[Google Scholar]
|
| 18. |
vanBijsterveld OP. Diagnostic tests in the sicca syndrome. Arch Ophthalmol 1969;82:10-4.
[Google Scholar]
|
| 19. |
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000;118:615-21.
[Google Scholar]
|
| 20. |
Gladman DD. Psoriatic arthritis. DermatolTher 2009;22:40-55.
[Google Scholar]
|
| 21. |
Jamshidi F, Bouzari N, Seirafi H, Farnaghi F, Firooz A. The prevalence of psoriatic arthritis in psoriatic patients in Tehran, Iran. Arch Iran Med 2008;11:162-5.
[Google Scholar]
|
| 22. |
Montaudié H, Sbidian E, Paul C, Maza A, Gallini A, Aractingi S, et al. Methotrexate in psoriasis: A systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. J EurAcadDermatolVenereol 2011;25 Suppl 2:12-8.
[Google Scholar]
|
| 23. |
Lima FB, Abalem MF, Ruiz DG, Gomes Bde A, Azevedo MN, Moraes HV Jr., et al. Prevalence of eye disease in Brazilian patients with psoriatic arthritis. Clinics (Sao Paulo) 2012;67:249-53.
[Google Scholar]
|
| 24. |
Paiva ES, Macaluso DC, Edwards A, Rosenbaum JT. Characterisation of uveitis in patients with psoriatic arthritis. Ann Rheum Dis 2000;59:67-70.
[Google Scholar]
|
| 25. |
Queiro R, Torre JC, Belzunegui J, González C, de Dios JR, Unanue F, et al. Clinical features and predictive factors in psoriatic arthritis-related uveitis. In: Seminars in Arthritis and Rheumatism. Vol. 31. Philadelphia: WB Saunders; 2002. p. 264-70.
[Google Scholar]
|
| 26. |
Oram Y, Akkaya AD. Treatment of nail psoriasis: Common concepts and new trends. Dermatol Res Pract 2013;2013:180496.
[Google Scholar]
|
| 27. |
Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004;23:762-70.
[Google Scholar]
|
| 28. |
Scotto di Fazano C, Grilo RM, Vergne P, Coyral D, Inaoui R, Bonnet C, et al. Is the relationship between spondyloarthropathy and Sjögren's syndrome in women coincidental? A study of 13 cases. Joint Bone Spine 2002;69:383-7.
[Google Scholar]
|
| 29. |
Lemp MA. Dry eye (keratoconjunctivitissicca), rheumatoid arthritis, and Sjögren's syndrome. Am J Ophthalmol 2005;140:898-9.
[Google Scholar]
|
| 30. |
Fujita M, Igarashi T, Kurai T, Sakane M, Yoshino S, Takahashi H. Correlation between dry eye and rheumatoid arthritis activity. Am J Ophthalmol 2005;140:808-13.
[Google Scholar]
|
Fulltext Views
5,220
PDF downloads
4,501





