Translate this page into:
Primary cutaneous marginal zone B-cell lymphoma of vulva in a pregnant woman
Correspondence Address:
Lin Wang
No. 37, Guo Xue Xiang, Wuhou District, Chengdu 610041, Sichuan Province
China
| How to cite this article: Tang J, Chen J, Liu H, Wang L. Primary cutaneous marginal zone B-cell lymphoma of vulva in a pregnant woman. Indian J Dermatol Venereol Leprol 2019;85:211-214 |
Sir,
A 36-year-old Chinese pregnant woman (gravida 2, para 1, at 7 weeks gestation) presented to the hospital with a progressive painful ulcerated mass on the vulva which had appeared 2 months back. The mass started as a tender nodule of about 2 cm in diameter and progressed irrespective of intravenous benzylpenicillin and debridement. The patient denied any fever, night sweats, weight loss or other systemic symptoms. Her previous medical history and family history were unremarkable.
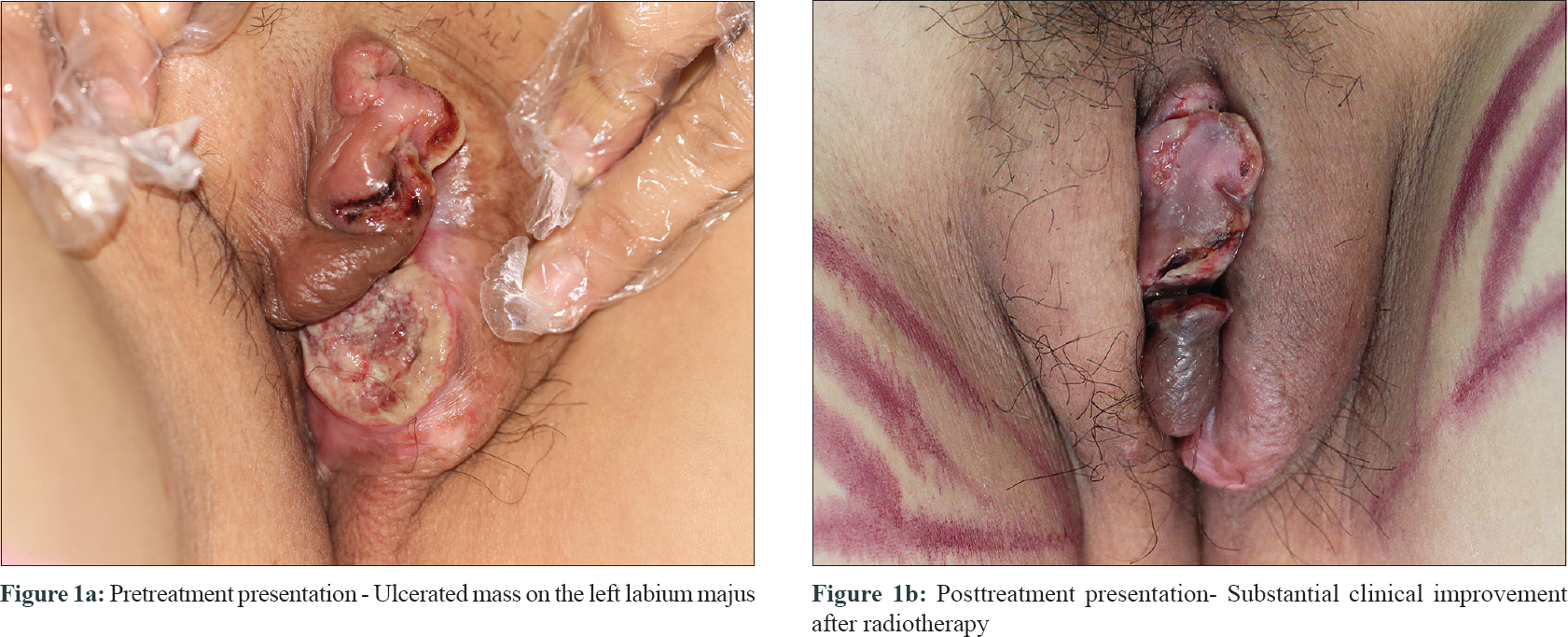
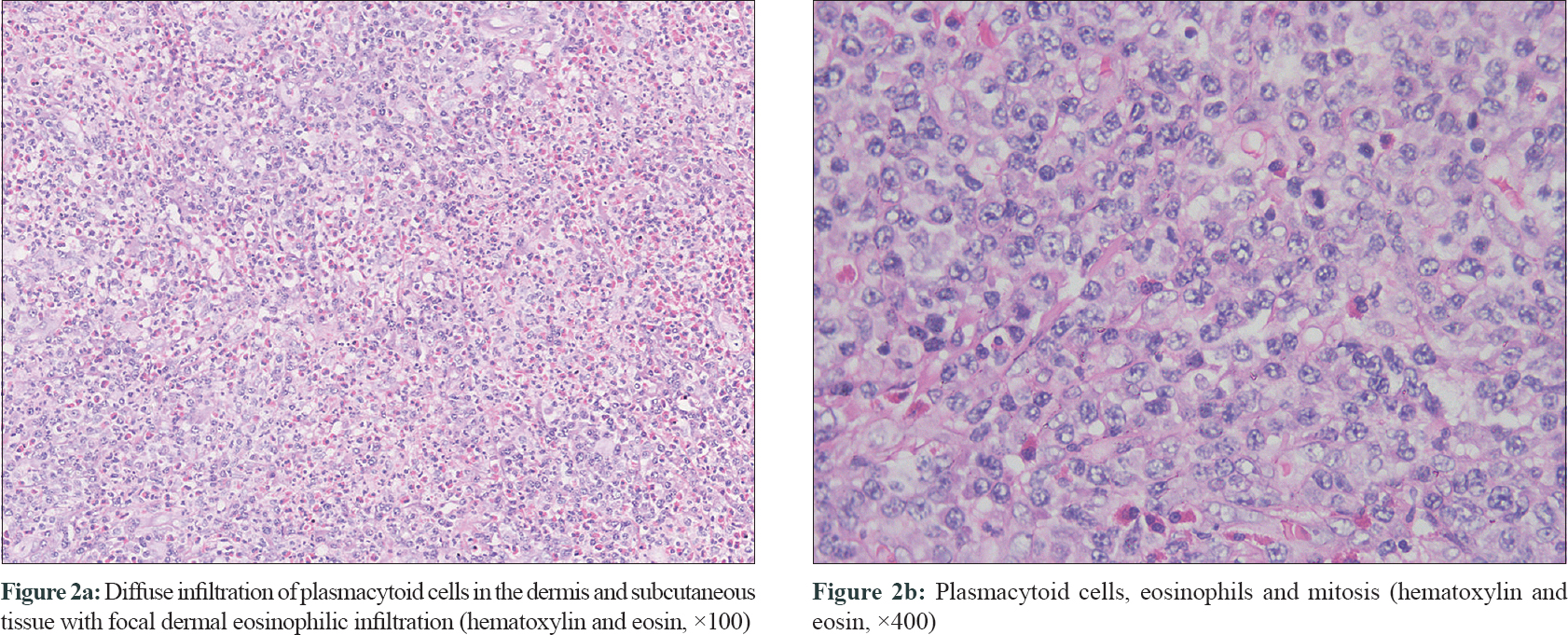
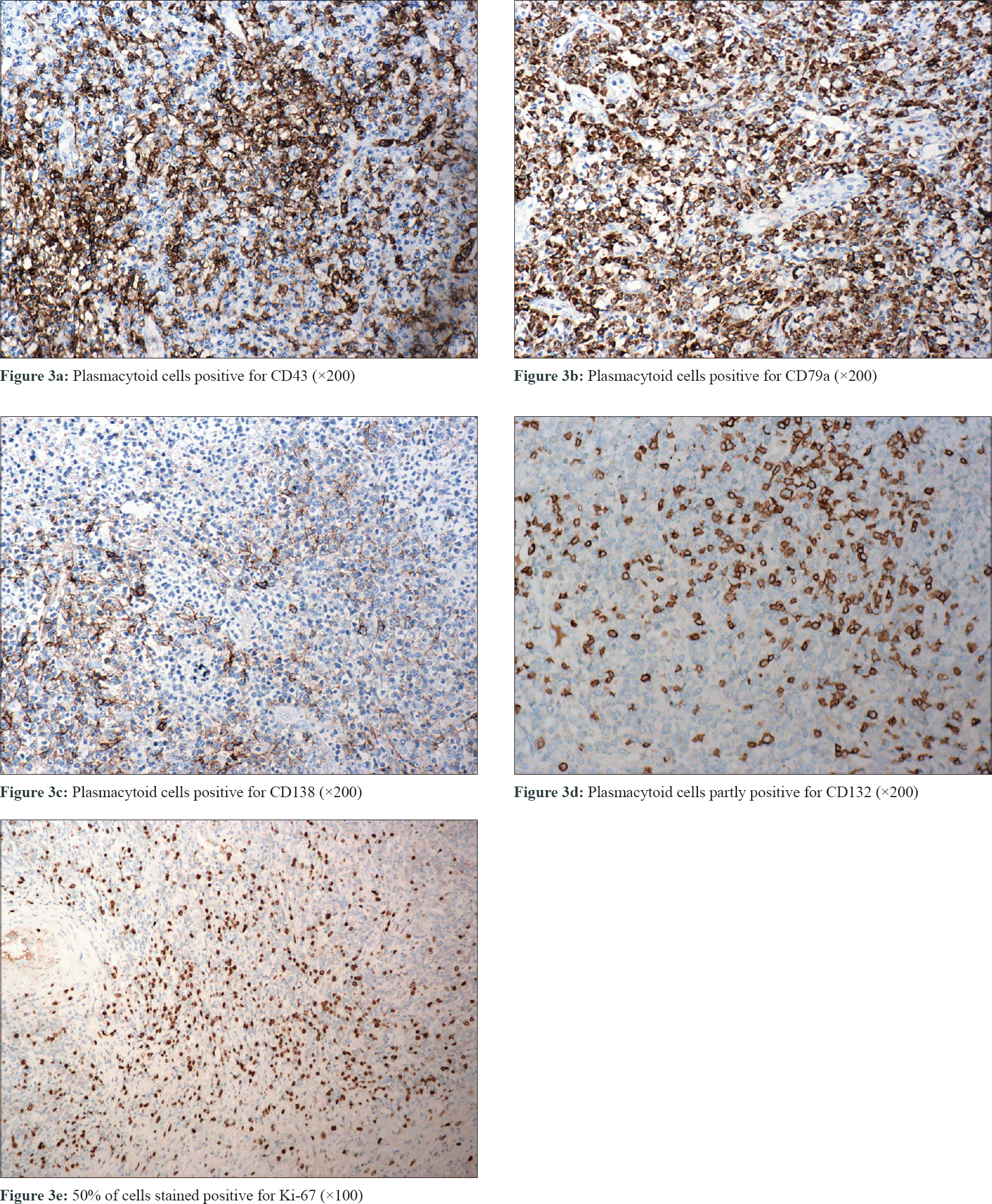
Physical examination of her left labium majus revealed an irregular, ill-circumscribed, ulcerated mass measuring 4 cm × 7 cm with hemorrhagic crusts and purulent discharge [Figure - 1]a. The inguinal lymph nodes were not palpable. Laboratory tests revealed a low hemoglobin level (99 g/L). Bone marrow aspiration and biopsy were unremarkable except for hyperplastic anemia. Magnetic resonance imaging found an irregular mass located in the vulva with mixed signals. Serological tests of syphilis and human immunodeficiency virus, bacterial culture of the lesional samples and computed tomography inspection of chest and abdomen were noncontributory. A biopsy was performed from the edge of the lesion. Histopathologic examination [Figure - 2] revealed partially absent epidermis and diffuse infiltration of plasmacytoid cells in the dermis and subcutaneous tissue. Focal dermal eosinophilic infiltration, mitosis (0-3/HPF) and scattered necrosis were also noticed. The plasmacytoid cells were positive for CD43, CD79a, CD138 and κ-light chain, partly positive for CD132 [Figure - 3]a, [Figure - 3]b, [Figure - 3]c, [Figure - 3]d, and negative for CD3, CD5, CD10, CD20, CD23, B-cell lymphoma (Bcl)-2, Bcl-6 and λ-light chain. The percentage of Ki-67 positively stained cells was about 50% [Figure - 3]e. These findings were consistent with primary cutaneous marginal zone B-cell lymphoma.
 |
| Figure 1: |
 |
| Figure 2: |
 |
| Figure 3: |
After terminating the pregnancy with induced abortion, the patient was successfully treated with daily fractionation of 200 cGy in five fractions weekly (21 fractions in total) [Figure - 1]b, except for the third fraction in which 500 cGy was adopted because of aggravated pain. An extended local excision of two small remnant nodules in the vulva was performed 40 days after the discontinuance of fractionation. The postoperative pathology revealed no tumor cells. No evidence of recurrence or metastasis was noted during 3-year follow-up period.
Primary cutaneous marginal zone B-cell lymphoma is a low-grade malignant B-cell lymphoma and has been included within the group of extranodal marginal zone lymphoma of the mucosa-associated lymphoid tissue in the World Health Organization classification.[1],[2]
The clinical presentations of primary cutaneous marginal zone B-cell lymphoma are characterized by papules, nodules, plaques or tumors located on trunk or upper extremities.[2],[3] It affects middle-aged individuals with a male predominance.[3] This case illustrates the rarity of the location of lymphoma, the site being vulva, the presentation as an ulcerated tumor and the concomitance of pregnancy. Although no evidence suggests a causative association between pregnancy and lymphoma, hormone-related receptors and the immunosuppressive milieu characterizing pregnancy may accelerate tumor progression.[4] Review of PubMed, Google Scholar and Web of Science by using the search terms “primary cutaneous marginal zone B-cell lymphoma” AND “ulcer” or “primary cutaneous marginal zone lymphoma” AND “ulcer” indicates that this case might be the initial report of primary cutaneous marginal zone B-cell lymphoma with ulcerative lesion.
Four main histopathologic presentations of primary cutaneous marginal zone B-cell lymphoma are recognized despite overlapping features. The plasmacytic variant, formerly named cutaneous plasmacytoma, is proposed for this case. Histopathologically, plasmacytic variant shows dense nodules and/or sheets of cells within the entire dermis and subcutaneous tissue, predominantly composed of mature plasma cells admixed with some blastoid cells.[5] Most B-cell-associated markers are negative, but cells can be stained by antibodies specific for CD38, CD132 or sometimes CD79a.
With respect to an ulcerated tumor on vulva, various malignancies and infections should be included in the differential diagnosis of primary cutaneous marginal zone B-cell lymphoma, such as Langerhans cell histiocytosis, squamous cell carcinoma and tuberculosis cutis orificialis. Langerhans cell histiocytosis can present at any age with a predominance in 0–4 years age group. Langerhans cell histiocytosis in adults may be papular, pustular or nodular lesions and ulceration of the flexures, groin, perianal area or vulva is common.[6] The diagnosis of Langerhans cell histiocytosis is based on predominant infiltration of Langerhans cells with expression of CD1a, S100 and Langerin.[7] Squamous cell carcinoma is a malignant tumor arising from epidermal keratinocytes or its appendages, which is diagnosed histologically based on the presence of either descending strands of morphologically malignant keratinocytes or single atypical keratinocytes.[6] Tuberculosis cutis orificialis often affects middle-aged adults and elderly presenting with advanced visceral tuberculosis or impaired cellular immunity. Lesions appear as painful papules and nodules that can lead to ulcers on the skin near orifices. In conclusion, a combination of clinical presentation, histological findings and molecular genetics confirms the diagnosis of primary cutaneous marginal zone B-cell lymphoma.
It has been confirmed that radiotherapy and surgery are the most frequently used therapies in primary cutaneous marginal zone B-cell lymphoma.[2] In this case, radiotherapy was supplemented with surgery on the remnant lesion, leading to a complete response without recurrence. The prognosis of this entity is excellent with a median disease-free survival of 47 months, an overall survival at 5 and 10 years of 93% and <1% patients dying of the lymphoma progression.[2]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: An overview. Pathologica 2010;102:83-7.
[Google Scholar]
|
| 2. |
Servitje O, Muniesa C, Benavente Y, Monsálvez V, Garcia-Muret MP, Gallardo F, et al. Primary cutaneous marginal zone B-cell lymphoma: Response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol 2013;69:357-65.
[Google Scholar]
|
| 3. |
Hoefnagel JJ, Vermeer MH, Jansen PM, Heule F, van Voorst Vader PC, Sanders CJ, et al. Primary cutaneous marginal zone B-cell lymphoma: Clinical and therapeutic features in 50 cases. Arch Dermatol 2005;141:1139-45.
[Google Scholar]
|
| 4. |
Brenner B, Avivi I, Lishner M. Haematological cancers in pregnancy. Lancet 2012;379:580-7.
[Google Scholar]
|
| 5. |
Cerroni L, editor. Cutaneous marginal zone lymphoma (cutaneous MALT-lymphoma) and variants. In: Skin Lymphoma: The Illustrated Guide. 4th ed. Oxford: Wiley-Blackwell Ltd.; 2014. p. 201-19.
[Google Scholar]
|
| 6. |
Gupta G, Madan V, Lear JT. Squamous cell carcinoma and its precursors. In: Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook's Textbook of Dermatology. 9th ed. Oxford: John Wiley & Sons Ltd.; 2017. p. 3955-60.
[Google Scholar]
|
| 7. |
Grana N. Langerhans cell histiocytosis. Cancer Control 2014;21:328-34.
[Google Scholar]
|
Fulltext Views
3,560
PDF downloads
2,002





