Translate this page into:
Primary mucinous carcinoma of skin with a trichoadenomatous component: A rare case report
2 Leelavathi Advanced Skin and Laser Centre, Guntur, Andhra Pradesh, India
Correspondence Address:
Chennamsetty Kavya
Leelavathi Advanced Skin and Laser Centre, Anna Srinivasarao Kalyanamandapam Road, Kothapeta, Guntur, Andhra Pradesh
India
| How to cite this article: Kavitha A, Kavya C, Sneha K, Teja C. Primary mucinous carcinoma of skin with a trichoadenomatous component: A rare case report. Indian J Dermatol Venereol Leprol 2019;85:171-174 |
Abstract
Primary mucinous carcinoma of the skin is a rare subtype of eccrine sweat gland tumors. Differentiating it from metastatic adenocarcinomas is important in the management of this condition. We report the case of a 55-year-old female presenting with a painless nodule, which was subsequently diagnosed as primary mucinous carcinoma of skin with a trichoadenomatous component. The possibility of a metastatic adenocarcinoma was ruled out by performing ultrasound abdomen, total body computed tomography, mammogram and colonoscopy.
Introduction
Primary mucinous carcinoma of the skin, a rare subtype of eccrine sweat gland tumor based on immunohistochemical and electron microscopic evidence, was first described by Lennox et al. in 1952[1] and was formally reviewed by Mendoza and Helwig in 1971.[2] Differentiating this condition from metastatic adenocarcinomas is crucial in the management of this condition.
Case Report
A 55-year-old female presented with a 5-year history of a painless nodule on the right zygomatic area. On examination, a skin colored nodule with a smooth surface without telangiectasia or ulceration, measuring about 5 × 5 mm, was noted [Figure - 1]. Regional lymph nodes were not palpable.
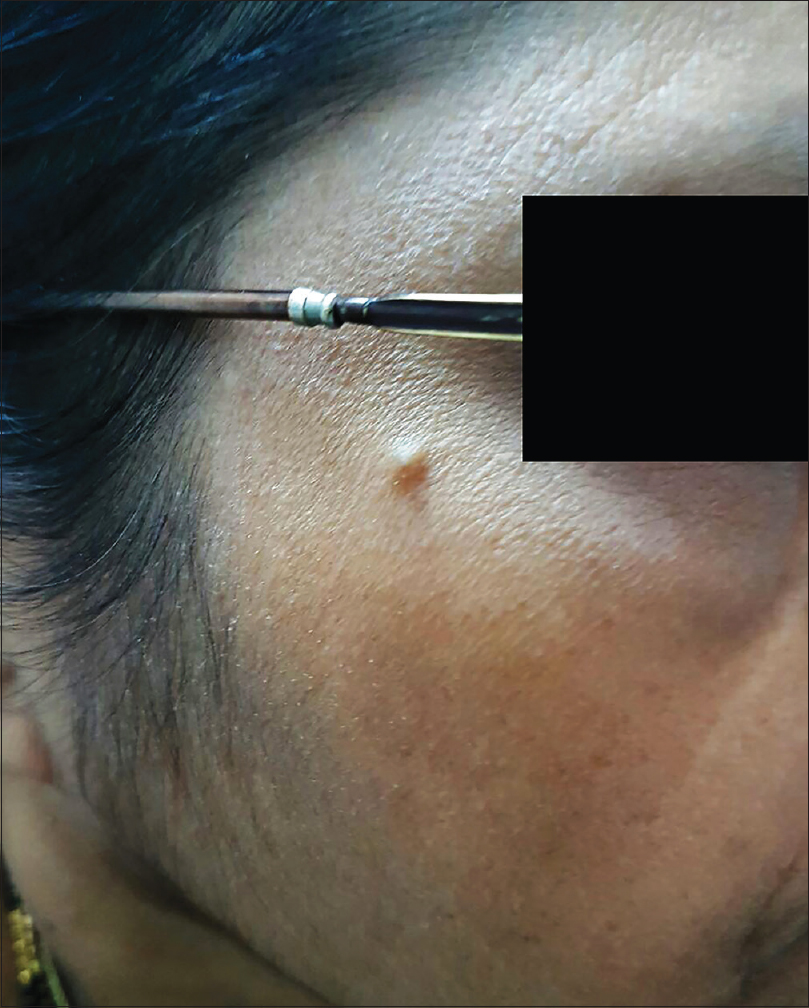 |
| Figure 1: Skin-colored, smooth-surfaced nodule of 5 × 5 mm size on the right zygomatic area |
Histopathological evaluation of the excised lesion revealed numerous keratinous cysts containing vellus hair in mucinous stroma within the upper dermis, separated from the mid and lower dermis [Figure - 2]a and [Figure - 2]b. Large pools of basophilic mucin separated by thin fibrovascular septa with floating islands of cohesive epithelial cells in solid and cribriform pattern were present in the mid dermis and subcutis. No significant mitoses were seen [Figure - 3]. Immunohistochemistry revealed cytokeratin (CK)-7, estrogen receptor and progesterone receptor positivity, smooth muscle actin, S-100P focal positivity and weak calponin positivity. CK-20, CDX2, anti-P63 were negative, favoring the diagnosis of primary mucinous carcinoma of skin [Figure - 4]. Investigations were done to rule out primary visceral malignancies; including ultrasound abdomen, total body computed tomography (CT), mammogram and colonoscopy, which were negative. The patient was treated surgically with excision of the tumor with a 1 cm margin and is on follow-up for the past 6 months, with no recurrence.
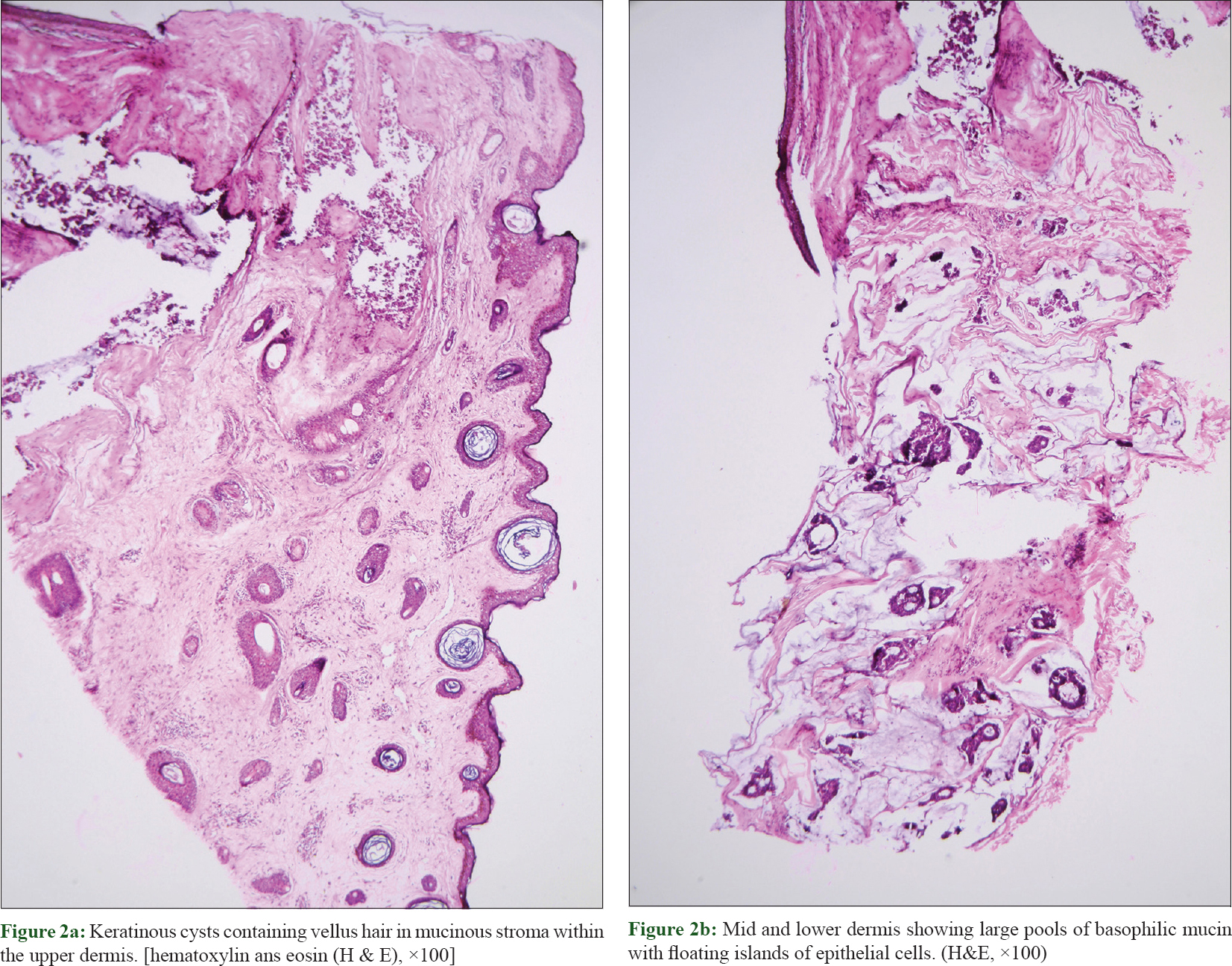 |
| Figure 2: |
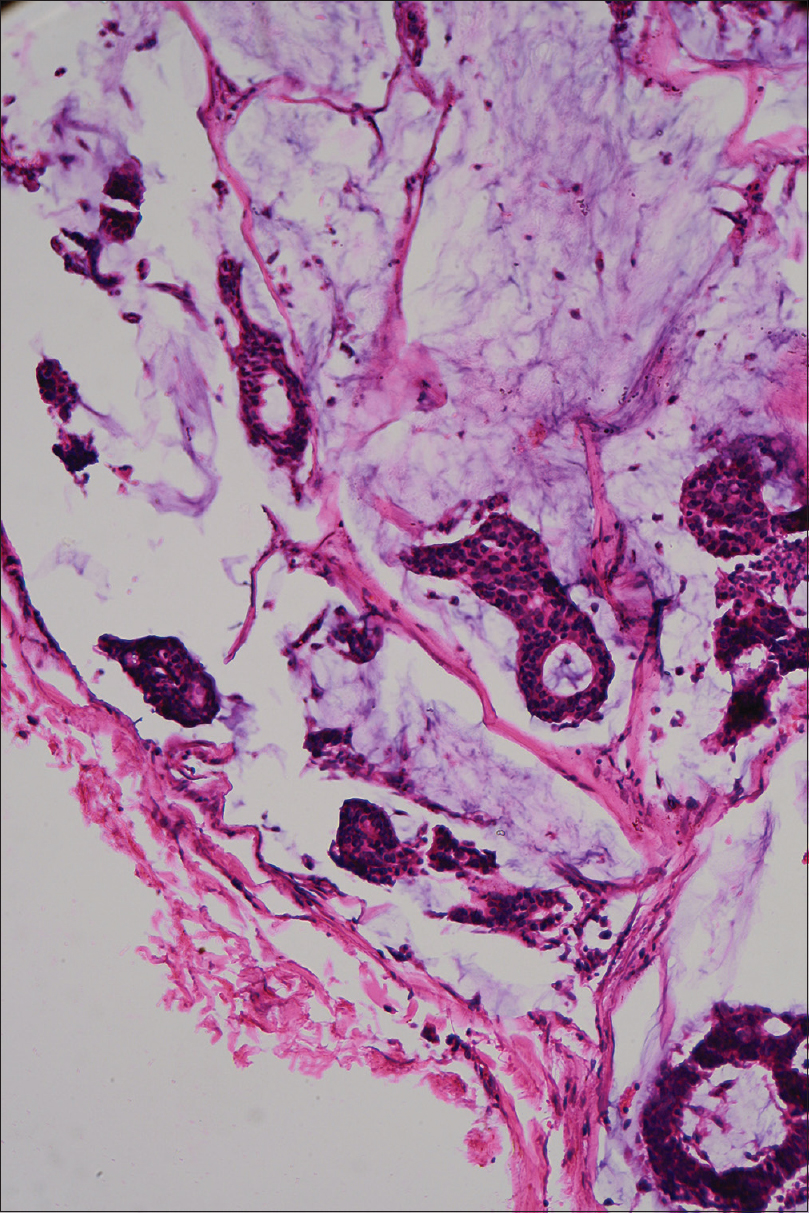 |
| Figure 3: Cords of epithelial cells in solid and cribriform pattern floating in basophilic mucin and seperated by fibrovascular septa (H&E, ×100) |
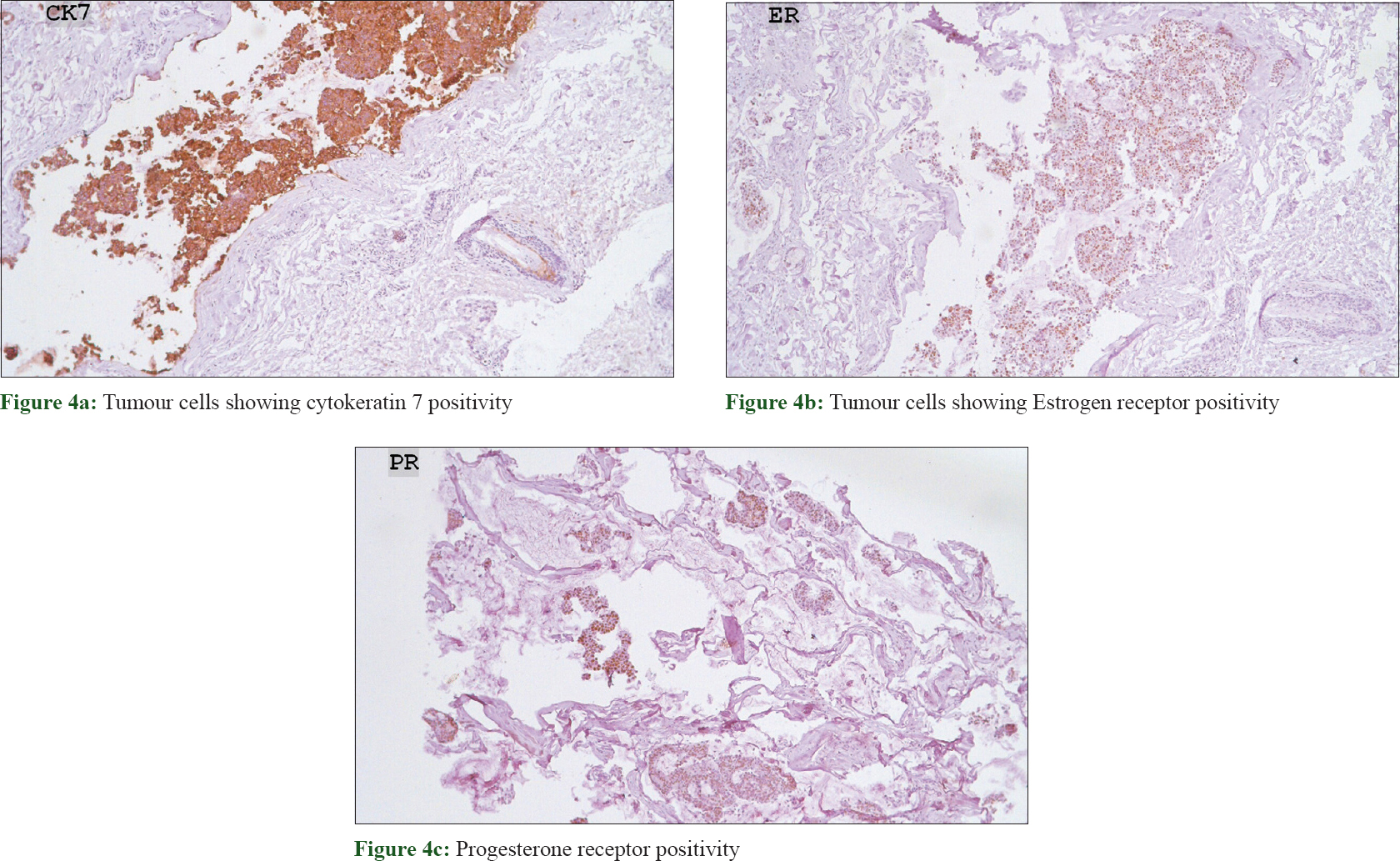 |
| Figure 4: |
Discussion
Primary mucinous carcinoma of the skin is a very low-grade malignancy of the eccrine sweat glands.[3] Men are two times more commonly affected than women. The condition favors the head and neck region; eyelids being the most common site, followed by the scalp, face, axilla, vulva, chest, abdominal wall, neck, extremities, canthus, groin and ear.[3]
The tumor typically presents as an isolated, slow-growing, asymptomatic, painless, papular, nodular or subcutaneous growth ranging in size from 5 to 120 mm. The surface may be smooth, ulcerated or crusted and may or may not show telangiectasia.[4]
The histopathological features of primary mucinous carcinoma of skin include cords and nests of basaloid cells embedded in a pool of periodic-acid Schiff and colloid iron positive mucin, separated by thin fibrous septa.[5] Rosai hypothesized that the island of tumor cells floating in mucinous pools is due to ductal hyper-proliferation, leading to overproduction of mucin.[6] Mucin in primary mucinous carcinoma of skin is a non-sulfated mucoprotein, most likely sialomucin, and hence is hyalorunidase-resistant, alcian blue positive at pH 2.5 and negative at pH 0.4, in contrast to the gastrointestinal tumors which produce sulfamucins (alcian blue positive at pH 1.0 and 0.4). Eccrine origin of the tumor is supported by enzyme histochemistry with succinic dehydrogenase, lactic dehydrogenase and isocitric dehydrogenase positivity.[7]
Immunohistochemistry helps in differentiating primary mucinous carcinoma of the skin from metastatic adenocarcinomas from the breast, gastrointestinal tract, ovary, prostate, lung etc., This condition demonstrates consistently positive staining with low molecular weight cytokeratins and epithelial membrane antigens. P63/SMA staining helps in identifying the myoepithelial component that is present only in cutaneous tumors. Carcinoembryonic antigen (CEA) and S-100 protein expression is variable.[3],[8] Estrogen and progesterone receptor expression in primary mucinous carcinoma of skin is indistinguishable from mucinous adenocarcinoma arising in the breast with p-53 and c-erb-2 negativity.[3] Breast carcinomas are typically positive for GCDFP-15 and often for estrogen receptors, while negative for vimentin. Colonic carcinomas show prominent positivity for CEA and CK-20, while no staining is seen for estrogen receptor and vimentin.[9]
The clinical mimickers include sebaceous cyst, amelanotic melanoma, lymphoma and Merkel's tumors. Adenocystic basal cell carcinoma, poorly differentiated squamous cell carcinoma and metastatic adenocarcinomas are histopathological mimickers. Mucinous metastasis from colon and breast carcinoma to the skin are most commonly found on the anterior chest wall rather than the head and neck, where most primary skin tumors occur. Thorough evaluation of patients with breast examination, mammogram, total body CT scan and colonoscopy is mandatory to rule out visceral carcinomas and to diagnose primary carcinoma of skin.
Treatment of primary mucinous carcinoma of the skin is wide local excision with a 1 cm margin. Tumor is unresponsive to radiation or chemotherapeutic agents.[10] Regional lymph node resection is indicated only if nodes are clinically involved. Distant metastasis is rare because of the avascular nature.[11] However, local recurrences are common (29.4%) and hence close follow-up is warranted.
The presence of a follicular (trichoadenomatous) component in our case is intriguing. The presence of follicular/epithelial component within sweat gland tumors is usually suggestive of a mixed tumor (chondroid syringoma).[12] However, the histological features in our case do not favor a mixed tumor as the stromal component is most often chondromyxoid, hyalinized/fibrous, fatty, osteoid or may even be minimal or absent in a classical chondroid syringoma.[13] Perhaps follicular/epithelial induction differentiation within the primary mucinous carcinoma may explain the presence of a trichoadenomatous component within the tumor. We have not found such an occurrence previously described in the literature.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Acknowledgement
- Dr. P. Ramana Kumari, Consultant pathologist, RHP Lab, Guntur, Andhra Pradesh, India
- Dr. Sasi Kiran Athili, Dermatologist, Cosmetic surgeon and Dermatopathologist, Visakha Institute of Skin and Allergy, Visakhapatnam, Andhra Pradesh, India.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Lennox B, Pearse AG, Richards HG. Mucin-secreting tumours of the skin with special reference to the so-called mixed-salivary tumour of the skin and its relation to hidradenoma. J Pathol Bacteriol 1952;64:865-80.
[Google Scholar]
|
| 2. |
Mendoza S, Helwig EB. Mucinous (adenocystic) carcinoma of the skin. Arch Dermatol 1971;103:68-78.
[Google Scholar]
|
| 3. |
Martinez S, Young S. Primary Mucinous Carcinoma of the Skin: A Review. Internet J Oncol 2004;2:2.
[Google Scholar]
|
| 4. |
Scilletta A, Soma PF, Grasso G, Scilletta R, Pompili G, Tarico MS, et al. Primary cutaneous mucinous carcinoma of the cheek. Case report. G Chir 2011;32:323-5.
[Google Scholar]
|
| 5. |
Bellezza G, Sidoni A, Bucciarelli E. Primary mucinous carcinoma of the skin. Am J Dermatopathol 2000;22:166-70.
[Google Scholar]
|
| 6. |
Rosai J. Ackerman's Surgical Pathology. 7th ed. St. Louis, MO: Mosby; 1989. p. 1232-3.
[Google Scholar]
|
| 7. |
Headington JT. Primary mucinous carcinoma of skin: Histochemistry and electron microscopy. Cancer 1977;39:1055-63.
[Google Scholar]
|
| 8. |
Carson HJ, Gattuso P, Raslan WF, Reddy V. Mucinous carcinoma of the eyelid. An immunohistochemical study. Am J Dermatopathol 1995;17:494-8.
[Google Scholar]
|
| 9. |
Lagendijk JH, Mullink H, van Diest PJ, Meijer GA, Meijer CJ. Immunohistochemical differentiation between primary adenocarcinomas of the ovary and ovarian metastases of colonic and breast origin. Comparison between a statistical and an intuitive approach. J Clin Pathol 1999;52:283-90.
[Google Scholar]
|
| 10. |
Yeung KY, Stinson JC. Mucinous (adenocystic) carcinoma of sweat glands with widespread metastasis. Case report with ultrastructural study. Cancer 1977;39:2556-62.
[Google Scholar]
|
| 11. |
Martinez S, Young S. Primary mucinous carcinoma of the skin: A review. Internet J Oncol 2004;2:2.
[Google Scholar]
|
| 12. |
Obaidat NA, Alsaad KO, Ghazarian D. Skin adnexal neoplasms – Part 2: An approach to tumours of cutaneous sweat glands. J Clin Pathol 2007;60:145-59.
[Google Scholar]
|
| 13. |
Kakinuma H, Miyamoto R, Iwasawa U, Baba S, Suzuki H. Three subtypes of poroid neoplasia in a single lesion: Eccrine poroma, hidroacanthoma simplex, and dermal duct tumor. Histologic, histochemical, and ultrastructural findings. Am J Dermatopathol 1994;16:66-72.
[Google Scholar]
|
Fulltext Views
3,864
PDF downloads
1,913





