Translate this page into:
Primary mucinous carcinoma of skin with psammoma bodies
Correspondence Address:
Debarshi Saha
Department of Pathology, Kasturba Medical College, Light House Hill Road, Mangalore - 575 001, Karnataka
India
| How to cite this article: Saha D, Khadilkar UN, Pai RR, Kumar A. Primary mucinous carcinoma of skin with psammoma bodies. Indian J Dermatol Venereol Leprol 2014;80:367-369 |
Sir,
Primary mucinous carcinoma of skin (PMCS), a rare malignant tumor of the sweat glands or their germinal structures was first described by Lennox et al., in 1952. [1] This tumor is usually of eccrine origin; [2] less commonly, it is of apocrine derivation. [3] We report the presence of psammoma bodies in PMCS, which is an exceptional phenomenon.
Our patient, a 55-year-old male, had a small (1.2 cm diameter) skin colored cystic nodule on his left cheek, below the lower eyelid, which had been present for 6 months. The lesion was excised with a margin of 1 cm.
Microscopy showed an atrophic epidermis overlying a dermal tumor with prominent wide lakes of mucin separated by thin fibrous septae. Floating in these lakes, were cribriform cohesive nests composed mainly of dark cuboidal cells with slight pleomorphism and some pale cells towards the centre. Within these nests, there were numerous tubules containing mucin [Figure - 1]b. Concentric lamellations, identified as psammoma bodies, were found both freely floating, as well as in the nests [Figure - 1]a and [Figure - 3]f. Occasional partially calcified hyaline globules were seen within glandular lumina [Figure - 2]e.
 |
| Figure 1: (a) Numerous psammoma bodies in the tumor cell nests as well as in the mucin (H and E, ×100) (b) Dark cuboidal tumor cell nests floating in mucin (H and E, ×100) (c) Mucin stains with Alcian blue at pH 2.5 (×100) |
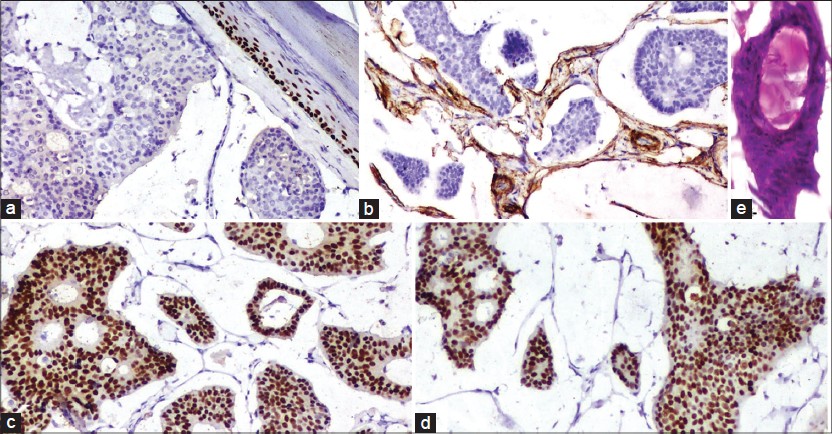 |
| Figure 2: (a) P63 - negative in the tumor cells (×100). (b) Smooth muscle actin - positive in the spindled cells lining the fibrous septae in the tumor (×100). (c) Estrogen receptor - strongly positive in the tumor cells (×100). (d) Progesterone receptor - strongly positive in the tumor cells (×100). (e) Partially calcified hyaline globule in a glandular lumen (H and E, ×100) |
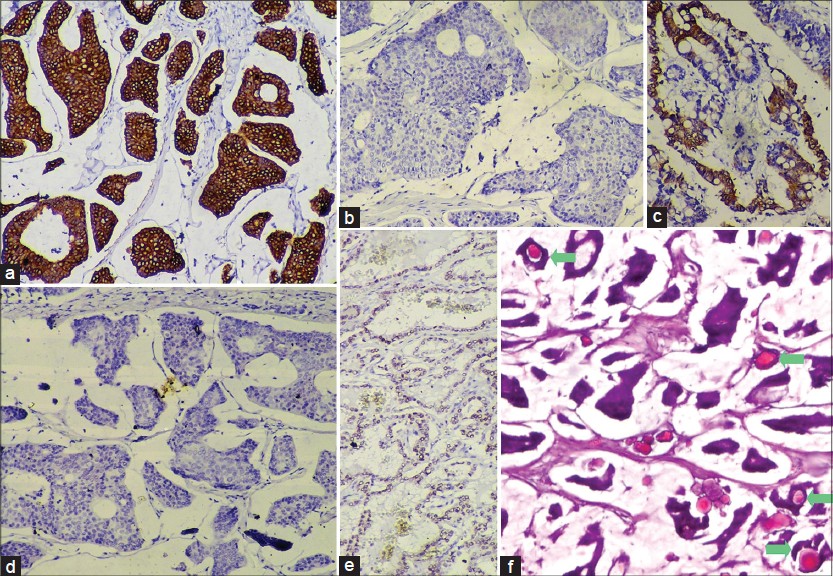 |
| Figure 3: (a) Cytokeratin 7 (CK7) - strongly positive in the tumor cells (×100). (b) CK20 – negative in tumor cells (×100) (c) CK20 -positive in control slide of colonic adenocarcinoma (×100). (d) Thyroid transcription factor-1 (TTF-1) - negative in tumor cells (×100). (e) TTF- 1 - positive in control slide of thyroid follicular cells (×100). (f) Psammoma bodies inside glandular lumina in the tumor (marked by green arrows, H and E, ×100) |
The mucin stained positively for periodic acid-Schiff and Alcian blue at pH 2.5 [Figure - 1]c suggesting non-sulfated sialomucin. Since metastatic mucinous carcinoma of the breast and other internal organs are included in the differential diagnosis, clinical and ultrasonologic breast examination, digital rectal examination of the prostate, abdominal ultrasound, colonoscopy, upper GI endoscopy and CT scan of the chest and neck were done, all of which were within normal limits. Follow-up 1½ years later showed a faint flushed scar and no evidence of any neoplasm.
While p63 was negative [Figure - 2]a, smooth muscle actin (SMA) was positive in the few flattened cells lining the fibrous septae [Figure - 2]b. The tumor cells stained strongly positive for estrogen [Figure - 2]c and progesterone [Figure - 2]d receptors (ER, PR). The tumor cells, although cytokeratin 7 (CK7) positive, were CK20 and thyroid transcription factor-1 negative [Figure - 3]a-e. Thus, a diagnosis of PMCS was made.
PMCS favors males (58.8%) over females (41%) and most commonly occurs in the head and neck region, with the eyelid being the preferred site (41%). [2] It usually presents as a painless, solitary, flesh colored to erythematous nodule of size varying from 3-4 mm to 20 cm, [1] usually not greater than 7-8 cm. [3] The histopathological appearance has been classically described as flotsam of dark tumor cells containing gland formations on faintly staining vast mucin lakes, partitioned by thin fibrous septae. [1],[2],[3] The histogenesis of this tumor has been suggested as a progression of abnormal eccrine or apocrine ducts, analogous to that of mucinous breast carcinoma. [4]
The differential diagnoses include mucinous carcinomas that metastasize to the skin arising from the breast, GI tract, respiratory tract, salivary and lacrimal glands, urinary tract, prostate and paranasal sinuses. [2] Of these, mucinous breast carcinomas stain positively for CK7 and may metastasize to the skin of the chest, breast and axilla, but rarely, the face. [1] The tumor cells of PMCS stain positively for low molecular weight cytokeratins (CK7, CAM 5.2), CEA, epithelial membrane antigen and S100 (inconstant). [3] The nuclei stain positively for ER and variably, PR, indicating its close histogenesis with that of mucinous breast carcinoma [3] and in our case, both were positive. However, an in situ component of the tumor may stain for CK5/6, p63, calponin and SMA on the myoepithelial cells and this may attest to the tumor being primary and not metastatic mucinous breast carcinoma. [5] In our case, p63 was negative but SMA was positive, indicating the presence of myoepithelial cells, thus, signifying a primary tumor rather than a metastatic one. GI carcinomas show CK20 positivity while PMCS is CK20 negative, but CK7 positive. [1],[3] GI carcinomas produce non-sulfated, neutral or sulfated mucins and not sialomucin, which is found in PMCS. [2] Metastatic colonic carcinomas may show dirty necrosis. [3]
Psammoma body formation is an active process where collagen fibers and membrane bound vesicles are laid down in concentric circles inside the papillary cores which are subsequently calcified. [6] Interestingly, papillary formations were absent in the present case. However, hyaline globules, which are the putative precursors of psammoma bodies [6] were present inside a few of the glandular lumina.
We found only one previous report of psammoma bodies in PMCS. [7]
| 1. |
Lennox B, Pearse AGE, Richards HGH. Mucin secreting tumors of the skin. With special reference to the so-called mixed salivary tumor of the skin and its relation to hidradenoma. Journal of Pathology and Bacteriology. 1952; 64:865-880.
[Google Scholar]
|
| 2. |
Martinez SR, Young SE. Primary mucinous carcinoma of skin: A review. Internet J Oncol 2005;2 (2) DOI: 10.5580/13e7.
[Google Scholar]
|
| 3. |
Calonje E, Brenn T, Lazar A, McKee PH. McKee's Pathology of the Skin with Clinical Correlation. 4 th ed., Vol. 2. Philadelphia: Elsevier Saunders; 2012. p.1566-8.
th ed., Vol. 2. Philadelphia: Elsevier Saunders; 2012. p.1566-8.'>[Google Scholar]
|
| 4. |
Kazakov DV, Suster S, LeBoit PE, Calonje E, Bisceglia M, Kutzner H, et al. Mucinous carcinoma of the skin, primary, and secondary: A clinicopathologic study of 63 cases with emphasis on the morphologic spectrum of primary cutaneous forms: Homologies with mucinous lesions in the breast. Am J Surg Pathol 2005;29:764-82.
[Google Scholar]
|
| 5. |
Qureshi HS, Salama ME, Chitale D, Bansal I, Ma CK, Raju U, et al. Primary cutaneous mucinous carcinoma: Presence of myoepithelial cells as a clue to the cutaneous origin. Am J Dermatopathol 2004;26:353-8.
[Google Scholar]
|
| 6. |
Das DK. Psammoma body: A product of dystrophic calcification or of a biologically active process that aims at limiting the growth and spread of tumor? Diagn Cytopathol 2009;37:534-41.
[Google Scholar]
|
| 7. |
Kalebi A, Hale M. Primary mucinous carcinoma of the skin: Usefulness of p63 in excluding metastasis and first report of psammoma bodies. Am J Dermatopathol 2008;30:510.
[Google Scholar]
|
Fulltext Views
3,570
PDF downloads
1,892





