Translate this page into:
Prolonged remission of pemphigus induced by dexamethasone-cyclophosphamide pulse therapy
Correspondence Address:
Ramji Gupta
47-C, Pocket B, Siddharth Extension, New Delhi - 110014
India
| How to cite this article: Gupta R. Prolonged remission of pemphigus induced by dexamethasone-cyclophosphamide pulse therapy. Indian J Dermatol Venereol Leprol 2007;73:121-122 |

Sir,
Since the introduction of dexamethasone-cyclophosphamide pulse (DCP) therapy for the treatment of pemphigus in 1982, [1] numerous workers at various centers have used it with good or satisfactory results. [2],[3],[4],[5],[6] Many have followed completely the four-phase regimen subsequently suggested. [7] A few have modified the same. [4] Majority of these workers have not reported duration of phase IV i.e., disease-free period without treatment and number of patients, if any, who developed relapse. Nor have they specified how many patients have completed five years or more in phase IV, which is very important before we claim that we have a regimen which is able to put pemphigus, a universally fatal disease, into prolonged or permanent remission. The purpose of this communication is to report long-term effect of DCP in pemphigus.
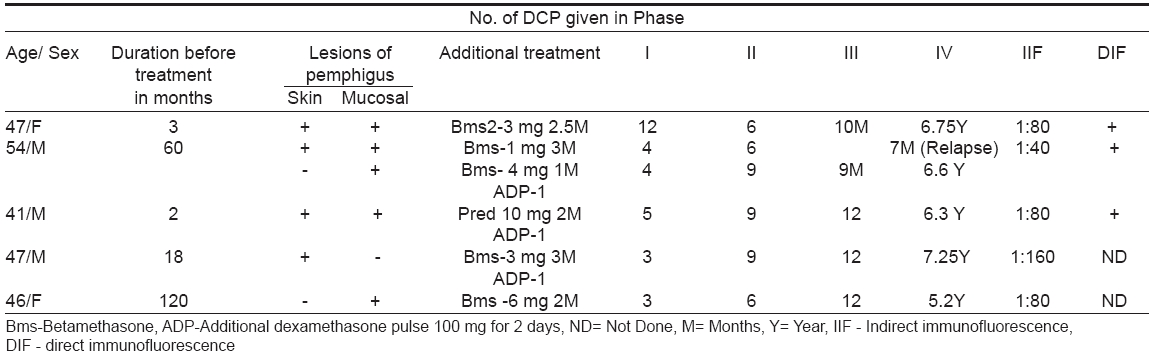
DCP therapy consists of transfusing 100 mg dexamethasone dissolved in 500 ml of 5% glucose over 1.5-2 hours, repeated on three consecutive days. On day one, the patient is also given cyclophosphamide 500 mg through the same drip. This is repeated at 28 days interval. In between, the patient receives 50 mg cyclophosphamide orally daily. This is called DCP therapy and is divided into four phases. In Phase I where duration is variable, the patient continues to develop recurrences of clinical lesions initially in between the DCPs, which subside with subsequent DCPs. In Phase II after the patient achieves complete clinical remission, he is given 9 more DCPs at intervals of four weeks. Cyclophosphamide 50 mg daily orally is continued in between the DCPs. In Phase III if patient is still in clinical remission, DCPs are stopped and patient takes only cyclophosphamide 50 mg orally daily for the next nine months. If the patient still continues to be in remission, cyclophosphamide is also withdrawn and patient is followed up for any relapse without any treatment for as many years as possible. This is called Phase IV. Before starting DCP, investigations done in all the patients include hemoglobin, total and differential leukocyte counts, platelets count, blood sugar, SGOT, SGPT, serum alkaline phosphatase, ECG, X-ray chest and weight charting. These investigations were repeated every one to two months. Skin biopsy and blood were also taken for immuno-fluorescence test to confirm the clinical diagnosis of pemphigus. Out of 10 patients of pemphigus who are being treated with DCP regimen by us, five who have been in continuous clinical remission for the last 6.5 to 9 years and without any treatment for the last 5 to 7 years are being reported. The rest have not yet crossed the five years of treatment.
Salient features of these five cases are shown in [Table - 1]. All the five patients (P. vulgaris-4, P. foliaceous-1) required additional betamethasone 1-6 mg daily in phase I along with DCP to cut short the duration of phase 1. Three patients also needed additional dexamethasone pulse, which consists of transfusing 100 mg dexamethasone dissolved in 500 ml 5% glucose over 1.5-2 hours, repeated on two consecutive days, at least 15 days after DCP is given. This helped in healing the lesions quickly and tapering the oral betamethasone faster. One patient developed relapse of mucosal lesions during the seventh month of phase III and was reverted back to phase I and treated accordingly with IV phase regime. Two patients received 6 DCPs in phase II while remaining three were given 9 DCPs. Phase III varies from 9-12 months where only 50 mg cyclophosphamide was given. All patients have been in continuous clinical remission for the last 6.5 to 9 years and without any treatment for the last five to seven years. Indirect immunofluorescence test was positive in all the five patients (1:40 to 1:80) before starting treatment, as also was direct immunofluorescence test done on skin of three patients. Two patients, in whom IIF and DIF were repeated after treatment, showed negative result at the end of phase III and 4.5 years in phase IV respectively.
Two patients noticed hiccups with few DCPs that were controlled in two to three days with prochlorperazine hydrochloride 5 mg thrice daily. One patient developed psychosis that was treated with imipramine. Two patients developed avascular necrosis of the femur neck 17 and 24 months after the last dose of DCP respectively and were treated by orthopedic surgeons. Invariably all four patients needed systemic antibiotics in phase I to control the secondary bacterial infections of the skin and those having oral ulcers (4 patients) needed oral fluconazole therapy till the clearance of oral candidiasis. However, no one required any oral antibiotics or antifungal medicines in phase II and III. One patient (patient no. 4) who was already having moon facies and obesity due to daily corticosteroids before coming to us, lost his excess weight and reverted back to his normal appearance, as treatment with DCP progressed. Generalized weakness and lethargy were reported by two patients following DCP, which were treated with 0.5 mg dexamethasone orally daily in three to four days. Metallic taste was noticed by three patients, which continued for four to five days after DCP.
The chief advantage of DCP is induction of quick remission with all the skin lesions improving within three to four days. The other advantage is freedom from the side-effects commonly seen with conventional daily doses of corticosteroids like weight gain, electrolyte imbalance, diabetes mellitus, hypertension, acne etc, which were hardly seen in the patients treated with DCP. The main problem with DCP is increased susceptibility to infections, bacterial as well as candidal in the phase I of the treatment when the skin and mucosae are eroded. However, both are controlled by giving suitable antibiotics and/or anti candida drugs. Another problem requiring attention is reactivation of tuberculosis, [1],[7] which can be treated by appropriate anti-tubercular treatment while DCP is being continued. Development of avascular necrosis of neck of femur 17 and 24 months in two patients after stoppage of DCP indicates that it is probably not directly due to DCP therapy [8] but is an independent post treatment phenomenon. Duration of posttreatment disease-free period has been more than five years (five to seven years) in all five patients.
A relapse after receiving complete and regular treatment or during treatment does not mean that DCP therapy has failed completely. A second course of DCP regimen in such cases usually achieves the desired results as happened to our second case who developed relapse during the seventh month in phase III and was subsequently treated with second course of DCP regime.
| 1. |
Pasricha JS, Gupta R. Pulse Therapy with dexamethasone cyclophosphamide in pemphigus. Indian J Dermatol Venereol Leprol 1984;50:199-203.
[Google Scholar]
|
| 2. |
Masood W, Hassan I, Majid I, Khan D, Manzooi S, Qayoom S, et al . Dexamethasone cyclophosphamide pulse therapy in pemphigus, experience in Kashmir Valley. Indian J Dermatol Venereol Leprol 2003;69:97-9.
[Google Scholar]
|
| 3. |
Narasimha Rao P, Laxmi TSS. Pulse therapy and it modification in pemphigus, a six-year study. Indian J Dermatol Venereol Leprol 2003;69:329-33.
[Google Scholar]
|
| 4. |
Kaur S, Kanwar AJ. Dexamethasone-cyclophosphamide pulse therapy in pemphigus. Int J Dermatol 1990;29:371-4.
[Google Scholar]
|
| 5. |
Roy R, Kalla G. Dexamethasone-cyclophosphamide pulse (DCP) therapy in pemphigus. Indian J Dermatol Venereol Leprol 1997;63:354-6.
[Google Scholar]
|
| 6. |
Sacchidanand S, Hiremath NC, Natraj HV, Revathi TN, Shobha Rani RH, Gupta P, et al . Dexamethasone-cyclophosphamide pulse therapy for autoimmune vesiculobullous disorders. Dermatol Online J 2003;9:2.
[Google Scholar]
|
| 7. |
Pasricha JS, Dass SS. Curative effect of dexamethasone cyclophosphamide pulse therapy for the treatment of pemphigus vulgaris. Int J Dermatol 1992;31:875-7.
[Google Scholar]
|
| 8. |
Fisher DE, Bickel WH. Corticosteroid induced avascular necrosis, a clinical study of seventy seven patients. J Bone Joint Surg 1971;53:859-73.
[Google Scholar]
|
Fulltext Views
2,664
PDF downloads
1,983





