Translate this page into:
Protective effect of bacillus Calmette Guιrin (BCG) vaccine in the prevention of leprosy: A meta-analysis
Correspondence Address:
Sanjay P Zodpey
A/303, Amar Enclave, Prashant Nagar, Ajni, Nagpur, Maharashtra
India
| How to cite this article: Zodpey SP. Protective effect of bacillus Calmette Guιrin (BCG) vaccine in the prevention of leprosy: A meta-analysis. Indian J Dermatol Venereol Leprol 2007;73:86-93 |
Abstract
Background: Although the role of bacillus Calmette Guιrin (BCG) vaccine in the prevention of leprosy was hypothesized as early as 1939, its level of protective effect remained controversial. Aim: As a meta-analysis systematically combines the results from different studies, we summarize the protective effect of BCG vaccine in prevention of leprosy using meta-analytic procedures. Methods: Our search strategy included a computerized literature search, snowballing technique to identify potential studies, review of previously compiled lists of BCG studies and articles, contacting experts on BCG vaccination and manual search to locate articles in non-indexed journals. The present meta-analysis included 22 studies (6 trials, 2 cohort studies and 14 case-control studies) on the role of BCG vaccine in the prevention of leprosy. The random effects model as described by DerSimonian and Laird was used to summarize the effect measures. For each summarization, a Chi-square test of heterogeneity was estimated. To strengthen the viewpoint further additional information from the studies which were not included in meta-analysis, was also utilized. Results: The summary protective effects calculated from trials, cohort studies and case-control studies were 43 (27-55), 62 (53-69) and 58 (47-67)% respectively, which were statistically significant. These estimates confirmed the protective association between BCG vaccination and leprosy. Review of 29 studies focusing on the role of BCG vaccination in the prevention of leprosy revealed that not a single study reported a negative protective effect. Thirteen (44.8%) studies demonstrated greater than or equal to 50% efficacy/effectiveness. Conclusion: There is sufficient and convincing evidence of the protective effect of BCG vaccine against leprosy, as reflected from the meta-analysis and overall review of 29 studies of BCG vaccination and leprosy.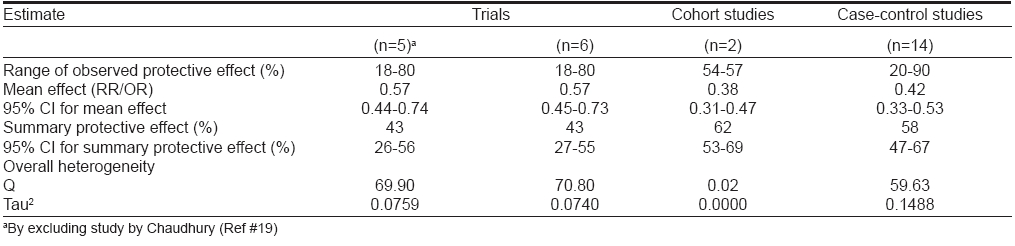




INTRODUCTION
The protective effect of bacillus Calmette Guιrin (BCG) vaccine in mycobacterial diseases has been a matter of long-standing controversy. Although the BCG vaccine has been rationalized for use in tuberculosis, its role in the prevention of leprosy was hypothesized as early as 1939. [1] These observations were later confirmed by several researchers [1],[2],[3] and led to a series of studies to evaluate the protective efficacy of BCG against leprosy. [4],[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17],[18],[19],[20],[21],[22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32],[33],[34],[35] The range of protection observed in these studies was very wide, i.e., 20-90%. [8],[22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32],[33],[34]
The variation in protective effect has been attributed to several factors: strain, dose and schedule of BCG vaccination; genetic and physiologic characteristics of the population; environmental mycobacteria and form of disease within the spectrum of leprosy. Moreover, different study designs, varying inclusion and exclusion criteria and different statistical estimators of the strength of association can all contribute to the variation in the estimated protective effect. As a consequence, summarization of the protective effect becomes difficult.
As meta-analysis systematically combines the results from different studies, we undertook to summarize the protective effect of BCG vaccine in prevention of leprosy using meta-analytic procedures.
METHODS
Identification of studies
A computerized literature search using the MEDLINE ® database was conducted. The following key words were used for searching: BCG, efficacy or effectiveness, protective effect and leprosy. The search was not limited to articles in the English language. Snowballing technique was then used to identify and locate potential studies by scanning the references of all the retrieved articles. Previously compiled lists of BCG studies and articles providing an overview of these studies were reviewed and experts on BCG vaccination were contacted. Manual search was also carried out to search for articles in non-indexed journals.
Inclusion and exclusion criteria
Only studies measuring the efficacy or effectiveness of BCG vaccination in preventing leprosy were included in the present meta-analysis. It included case-control studies, cohort studies as well as randomized control trials. Other observational studies (outbreak investigation, household contact study, surveillance study, case-series and incidence trends analysis) were also reviewed; however, these studies were not subjected to meta-analysis. Studies of leprosy prevalence, investigations of leprosy control programs and reviews of leprosy vaccination studies were searched for relevant references but were not included in the analysis. The studies that considered results of lepromin reaction as the outcome were excluded. Multiple reports of a single study were reviewed when they were available to obtain the most complete information possible. However, multiple reports did not amount to duplication of information; they were considered together as one single study. In case of several consecutive reports of the same study, the latest one was used for data extraction. By following these inclusion and exclusion criteria, the present meta-analysis finally included 22 studies on the role of BCG vaccine in prevention of leprosy.
Data extraction
The following information was extracted from each study: design of the study, authors, year of publication, number of participants enrolled (along with their exposure and disease status), location of study, population group and reported estimates of effect measures-relative risks / odds ratios (RR/OR) or vaccine effectiveness/ efficacy and their 95% confidence intervals (CI). When studies analyzed data using adjustments for a combination of variables (for example, using logistic regression or Poisson regression or Cox proportional hazards regression etc.), we recorded reported estimates of vaccine efficacy/effectiveness for an overall review. However for the purpose of meta-analysis, raw data (in the form of 2x2 tables) from each study was used as input data. When original studies did not report information in 2x2 tables, it was constructed using other available information in the study. This was necessary as the software [36] which was used for meta-analysis required information in the form of 2x2 tables as input data. Hence for a few studies, there is variation in estimates of OR/RR reported in original studies and calculated by Meta-Analyst software. [36]
Statistical analysis
The results are presented as relative risks (RRs) from the trials and cohort studies and as odds ratios (OR) from the case-control studies. The protective effect was also presented, as defined as 1-RR or 1-OR. The random effects model as described by DerSimonian and Laird [37] was used to summarize the effect measures. This model was separately applied to trials, cohort studies and case-control studies. For each summarization, a chi-square test of heterogeneity was estimated. The analysis was performed using Meta-Analyst software (Version 0.99 x; 1998). [36]
Review of studies
Although 29 studies were retrieved, which reported a protective effect of BCG in prevention of leprosy; meta-analysis could only include 22 studies, which satisfied the inclusion criteria. To strengthen the viewpoint further, the additional information from the studies not included in meta-analysis, was also utilized separately.
RESULTS
Twenty-nine studies of the protective effect of the BCG vaccine against leprosy were reported in literature. These studies were carried out by different investigators in different regions of the world and using different study designs [Table - 1]. Variation in the efficacy/effectiveness of BCG vaccine is evident from the results of these studies. The point estimates of protective effect obtained ranged from 18-90%.
When the results of an individual study were analyzed for significance of a protective effect (based on the point estimates and 95% CI), it was evident that five of 23 studies (six studies [10],[11],[12],[13],[14],[26] were not included for this analysis of significance as they did not report either point estimates or 95% CI) did not demonstrate any significant protective effect of BCG vaccination in the prevention of leprosy. Thus 18 (78.3%) studies recognized a significant protective association between BCG vaccination and leprosy.
Further analysis of these 29 studies according to the level of protection, revealed that not a single study reported a negative protective effect (point estimates for RR or OR > 1). A total of 16 (55.2%) studies exhibited less than 50% efficacy/effectiveness. However, about 12 (75%) of these 16 studies reported a protective effect in the range of 25-49%. Thirteen (44.8%) studies demonstrated ≥ 50% efficacy/effectiveness.
The same kind of analysis was also carried out by excluding five early trials, [10],[11],[12],[13],[14] which did not report point estimates but reported only the range of protection and had a major methodological limitation because of lack of comparability. After exclusion, studies that reported < 50% protective effects were brought down to 45.8%. A total of 13 (54.2%) studies demonstrated efficacy/effectiveness > 50%. Five (20.9%) studies reported ≥ 75% protection against leprosy.
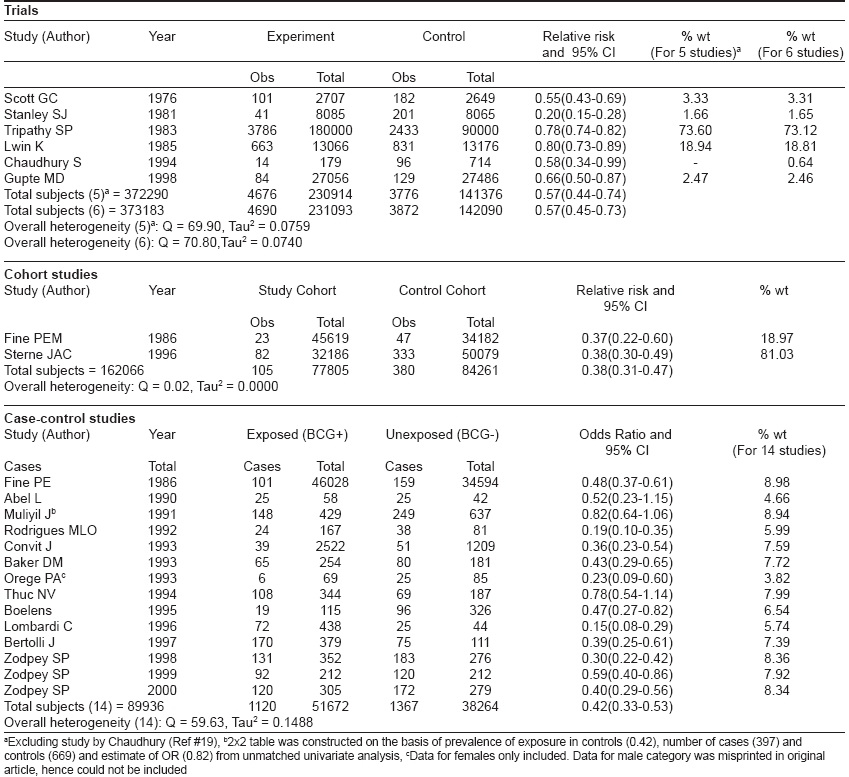
[Table - 1] describes the observed protective effect of BCG vaccines against leprosy as estimated in eight trials. [4],[5],[9],[15],[16],[17],[18],[19],[20],[21],[26] This table also includes the five early trials in Brazil, [10] Argentina, [11] Venezuela, [12] India [13] and Japan, [14] which demonstrated protection ranging from 26-96%. The point estimates for efficacy were not available for these studies and these early trials had several weaknesses and biases, particularly with regard to the comparability between BCG vaccinated and control groups. The range of protective effect (point estimates) observed in these trials was very wide, from 18-80% [Table - 1].
Of the above-described eight trials, six trials meeting the inclusion and exclusion criteria were subjected to meta-analysis using random effects model [Table - 2]. Venezuela trial, [26] which compared a BCG alone group with BCG + M. leprae group, was not included for meta-analysis because there was no comparison between the BCG-immunized and BCG-unimmunized groups in this trial. Additionally, the Northern Malawi trial (by Karonga Prevention Trial Group), [5] which evaluated the protective effect of ′repeat BCG′ in the prevention of tuberculosis was also not subjected to meta-analysis.
One study [19] reported a prospective trial of anti-leprosy vaccines in 893 household contacts of leprosy patients. Of these, 179 contacts were vaccinated using three different vaccine interventions, namely: killed M. leprae (n=85), BCG (n=46) and a mixture of M. leprae and BCG (n=48). While the study reported 14 new cases in the vaccine group within an eight year follow-up period, the distribution of these 14 leprosy cases among the three vaccine groups was not reported. The study, however, observed identical lepromin conversion rates across the three vaccine groups. Therefore, we included this study in the current meta-analysis with 179 and 714 subjects in the intervention and non-intervention groups, respectively. However, meta-analysis was also performed separately by excluding this study.
[Table - 2] describes the results of meta-analysis of trials using random effects model by including and excluding the study by Chaudhury. [19] By combining five trials, a summary relative risk of 0.57 (0.44-0.74) was estimated, which confirmed the significant protective association of BCG vaccination with leprosy. Addition of data from a sixth trial (as an independent and individual study) narrowed down the 95% CI slightly but the point estimate of 0.57 remained unaffected. These studies also demonstrated significant heterogeneity.
Two cohort studies [6],[7],[22] of BCG vaccination and leprosy were retrieved from literature. [Table - 1] shows the information extracted from these two studies. For one study, [6],[7] multiple reports were available for which the group with a doubtful BCG scar was merged with the BCG-negative group for calculating RR. This was necessary as the split-up information was not available for these groups. The two studies included in [Table - 1] reported moderate protective effect (57 and 54%) of BCG vaccination in the prevention of leprosy. Meta-analysis included these studies to estimate a summary protective effect using random effects model [Table - 2]. The DerSimonian and Laird random effects model estimated a weighted average RR of 0.38 (0.31-0.47) from the data of two cohort studies corresponding to a protective effect of 62% (53-69) against leprosy. The point estimate of protective effect was statistically significant as reflected by 95% CI. These studies did not report significant heterogeneity.
A total of 14 case-control studies of BCG vaccination and leprosy were reported in literature. [Table - 1] states the effectiveness, publishing authors, year of publication and study area for these studies. For one of these studies, multiple reports were available. The range of protection observed in these studies was very wide, i.e., 20-90%. All the 14 retrieved case-control studies were included in meta-analysis [Table - 2]. Combining the data from 14 case-control studies, a summary odds ratio (OR) of 0.42 (0.33-0.53) was estimated, which confirmed the significant protective association of BCG vaccination with leprosy. These studies also exhibited significant heterogeneity.
There was a slight variation in the estimates of OR (and thus vaccine effectiveness) reported in the original studies and obtained from the meta-analysis for a few case-control studies. Original studies reported OR based on different types of analysis i.e., matched, adjusted, stratified etc. However, for the purpose of meta-analysis using Meta-Analyst Software, the raw data (unmatched data from 2x2 tables) was used as input information as this was the requirement of statistical software. Therefore meta-analysis reported unmatched crude estimates of OR.
[Table - 3] summarizes the results of meta-analysis by study design. It is evident from the table that the summary RR from the cohort studies was lower than the summary OR from case-control studies and summary RR from trials. However, these differences were not statistically significant as suggested by overlapping 95% CI. The summary protective effects calculated from trials, cohort studies and case-control studies were 43 (27-55), 62 (53-69) and 58 (47-67)% respectively, which were statistically significant. These estimates confirmed the protective association between BCG vaccination with leprosy. Except cohort studies, the other two groups exhibited significant heterogeneity.
DISCUSSION
The story of vaccines against leprosy is unusual for its irony. Although some claim that there is no anti-leprosy vaccine, [38] more people alive today have received an anti-leprosy vaccine than have received any other vaccine. In fact, more than 100 million infants and children received it in 1995. The vaccine is BCG and the irony arises because the vaccine is so widely directed against tuberculosis that many have forgotten its important implications for leprosy. [39]
BCG was the first vaccine to be considered against leprosy following the report of Fernandez in 1939 on lepromin conversion among lepromin-negative healthy children following BCG administration. [40] The next 20 years witnessed a number of small-scale studies in selected populations, which suggested the value of BCG vaccine in the prevention of leprosy. [1],[17] A few early studies with methodologic limitations, demonstrated protection ranging from 26-96%. [10],[11],[12],[13],[14] Later in 1965, Shepard provided the first experimental evidence in favor of BCG in the mouse footpad model. [1] During the same decade, four major field trials were initiated in Uganda, [15] India, [2],[4],[20] Burma [17] and Papua New Guinea [9],[18] to evaluate the efficacy of BCG vaccine in the prevention of leprosy, which was followed by a few more trials. The results of these trials showed wide variation in the protective effect of BCG vaccine, ranging from 20-80%. [4],[9],[15],[16],[17],[18],[19],[20],[21],[41] A couple of cohort studies, which were carried out in Northern Malawi, exhibited 57 and 54% protective efficacy of the BCG vaccine. [6],[7],[22] Since P. G. Smith recommended the use of case-control studies for assessing vaccine effectiveness, a total of thirteen case-control studies of BCG vaccine effectiveness against leprosy were reported in literature. [8],[22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32],[33],[34],[42] The range of protection observed in these studies was also very wide, i.e., 20-90%. [8],[22],[23],[24],[25],[26],[27],[28],[29],[30],[31],[32],[33],[34],[42] Thus, the variations of efficacy against leprosy are reminiscent of variations observed of the effect of BCG against tuberculosis. [43]
In order to estimate the summary protective effect of BCG vaccine in prevention of leprosy, the meta-analysis of published studies of BCG vaccination and leprosy was carried out against this background. The summary protective effects calculated from trials, cohort studies and case-control studies were 43 (27-55), 62 (53-69) and 59 (46-68)% respectively, which were statistically significant and hence confirmed the protective association between BCG vaccination and leprosy. Thus, the findings of the current meta-analysis recognized a significant protective effect of BCG vaccination in the prevention of leprosy.
To substantiate the findings of current research work, 28 studies of BCG vaccination and leprosy were also reviewed. None of these studies reported a negative protective effect. Moreover, of the 22 studies (which reported significance either using 95% CI or P values), 17 (77.3%) studies had recognized a significant protective association between BCG vaccination and leprosy. A total of 12 (52%) studies out of 23 demonstrated a protective effect > 50%. Five (21.8%) studies reported > 75% protection against leprosy. This is certainly cumulative global evidence in favor of use of BCG vaccine for prevention of leprosy.
Few investigators went a step ahead to generalize the protective effect and stated that the BCG has had a demonstrable impact on the prevalence of leprosy despite variable results with BCG in formal studies. [44] Fine reviewed the reasons for declining trends of leprosy incidence throughout the world. [45],[46] Fine states that the leprosy community has long been schizophrenic on the subject of BCG. [45] The facts are simple. First, more people have received BCG than have received any other vaccine-till 1992 over two billion doses have been administered. Second, wherever it has been studied, it has been found that BCG imparts some protection against leprosy. The protection appears to be relatively modest in some contexts, for example Burma, [17] but quite high in others, for example, Eastern Africa. [15],[43] The implications of these two facts are simple-BCG must be having an impact on leprosy worldwide and is undoubtedly responsible, at least in part, for the decline in leprosy incidence observed in many populations. It is probably not a coincidence that the highest protective efficacy estimates for BCG against leprosy have come from studies in Africa (80% in Uganda) [15] and that some of the greatest recent decreases in incidence of leprosy have also been reported from that continent. [45]
While appreciating the role of BCG in leprosy prevention today, one should not neglect its idiosyncrasies, in particular, the fact that its effects differ between populations for reasons not understood so far. [43],[47] It is unfortunate that the effectiveness of BCG appears to be less in India than in Africa, given that there is much more leprosy in India than in Africa. And what about Brazil? Why is leprosy apparently increasing there in spite of socioeconomic improvement as well as widespread use of BCG? [45]
What is particularly important is that as far as available data is concerned, BCG vaccine is at least as effective in preventing leprosy as it is in preventing tuberculosis. In addition, the only three studies in which the activity of the same BCG vaccine in preventing leprosy and tuberculosis has been studied in the same populations, have actually demonstrated that the BCG vaccine is more effective against leprosy than against tuberculosis [54% vs 11% in Malawi; [48] 81% vs 22% in Kenya [8] and 22% vs -5% in South India]. [4],[16],[20],[49],[50],[51],[52]
This has important practical implications as well as implications for research. In practice, it means that at this moment, there is in progress a massive anti-leprosy vaccination program, which is undoubtedly playing an important role in the worldwide reduction of the incidence of leprosy. One should not therefore forget that when we talk of the low protective effect of BCG vaccine against tuberculosis, we also should take into account its role against leprosy. This is the additional advantage that we are gaining out of mass neonatal BCG vaccination programs. As we understand, although the BCG vaccination has not been an official or a formal component of Leprosy Control Programs anywhere in the world, it is still contributing to leprosy control. Secondly, the relationship between the actions of BCG in leprosy and in tuberculosis may provide a clue to the very important questions of protective immunity and correlates of protection against tuberculosis as well as leprosy. [10]
It is not surprising that while the BCG vaccine is being used for the prevention of tuberculosis, it is also preventing leprosy. The BCG vaccine is basically known to protect against disease caused by Mycobacterium tuberculosis , which has a number of antigens in common with M. leprae . There is convincing experimental and circumstantial evidence that exposure to one species of mycobacteria can provide an individual human host with some degree of protection against infection by another species. [53] BCG vaccination might therefore also protect against disease caused by M. leprae , i.e., leprosy. [9] The rationale of the use of BCG as a preventive vaccine against leprosy also rests on the assumption that cross-reacting antigens exist between M. leprae and BCG and that following BCG vaccination, protective immunity against leprosy will develop. [9]
The present study suffers from all the limitations that are inherent in any meta-analysis. The role of individual factors in explaining the observed heterogeneity could not be quantified. This was not possible because of the limitations of data extraction by many co-variates that might have contributed to variation. Another limitation of current meta-analysis is that when required, the effect measures and their CI were estimated using bi-variate analysis of 2x2 table. However, because different studies used different estimators of OR and RR, a simple summarization can mean an over-generalization of the individual study findings. In other words, by meta-analyzing, some information from individual studies might have been lost. An additional limitation of the current meta-analysis is that studies reporting sero-conversion after BCG immunization could not be included because outcomes from different studies would not have been compatible. Non-inclusion of unknown, unpublished studies can potentially bias the summary effects if the published literature shows a bias in either direction. To deal with this potential source of bias, unpublished literature of the protective effect of the BCG vaccine was extensively searched. However, unpublished studies which met selection criteria could not be located. Despite all the limitations, the present meta-analysis reported significant and stable estimates of protective effects across study designs.
Finally to conclude the discussion of this research, there is sufficient and convincing evidence of the protective effect of BCG vaccine against leprosy, as reflected from the meta-analysis of trials, cohort studies and case-control studies and overall review of 29 studies of BCG vaccination and leprosy. This cumulative evidence certainly strengthens the case of the usefulness of the BCG vaccine. In general, the results of the current study and review support arguments favoring the use of the BCG vaccine for the prevention of leprosy.
| 1. |
Shepard CC. Vaccination against experimental infection with M. leprae . Am J Epidemiol 1965;81:150-63.
[Google Scholar]
|
| 2. |
Convit J, Rassi E. Lepromin and tuberculin tests in Venezuelan leprosy foci: Induction of lepromin reaction by BCG vaccination. Int J Lepr 1954;22:303-10.
[Google Scholar]
|
| 3. |
Doull JA, Guinto RS, Mabalay MC. Effect of BCG vaccination, lepromin testing and natural causes in inducing reactivity to lepromin and tuberculin. Int J Lepr 1957;25:13-37.
[Google Scholar]
|
| 4. |
Tripathy SP. The case for BCG. Ann Natl Acad Med Sci 1983;19:11-21.
[Google Scholar]
|
| 5. |
Randomized controlled trial of single BCG, repeated BCG or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Karonga Prevention Trial Group. Lancet 1996;348:17-24.
[Google Scholar]
|
| 6. |
Ponnighaus JM, Fine PE, Sterne JA, Wilson RJ, Msosa E, Gruer PJ, et al . Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet 1992;339:636-9.
[Google Scholar]
|
| 7. |
Sterne JA, Fine PE, Ponnighaus JM, Sibanda F, Munthali M, Glynn JR. Does bacille Calmette-Guιrin scar size have implications for protection against tuberculosis or leprosy? Tuber Lung Dis 1996;77:117-23.
[Google Scholar]
|
| 8. |
Orege PA, Fine PE, Lucas SB, Obura M, Okelo C, Okuku P. Case-control study of BCG vaccination as a risk factor for leprosy and tuberculosis in Western Kenya. Int J Lepr 1993;61:542-9.
[Google Scholar]
|
| 9. |
Bagshawe A, Scott GC, Russell DA, Wingley SC, Merianos A, Berry G. BCG vaccination in leprosy: Final results of the trial in Karimui, Papua New Guinea, 1963-79. Bull WHO 1989;67: 389-99.
[Google Scholar]
|
| 10. |
DeSouza CN. Primary results of BCG in leprosy prophylaxis. Memoria del VI Congreso Internacional de Leprologia: Madrid; October 1953, p. 518-20 (In Spanish).
[Google Scholar]
|
| 11. |
Fernandez JM. Influence of the tuberculosis factor on the clinical and immunological evolution of child contacts with leprosy patients. Int J Lepr 1955;23:243-58.
[Google Scholar]
|
| 12. |
Convit J. Study on leprosy in the German ethnic group of Colonia Tovar, Venezuela. The morbidity rates in BCG vaccinated and unvaccinated groups during five years. Int J Lepr 1956;24: 269-74.
[Google Scholar]
|
| 13. |
Chatterjee KR, Soucou P, Sainte-Rose M. Prophylactic value of BCG vaccination against leprosy: A preliminary report. Bull Calcutta Sch Trop Med 1958;6:164-6.
[Google Scholar]
|
| 14. |
Yanagisawa K. On the immunological relationship between tuberculosis and leprosy with special reference to the effect of BCG administration upon the prophylaxis of leprosy. La Lepro 1960;26-28:37-47.
[Google Scholar]
|
| 15. |
Stanley SJ, Howland C, Stone MM, Sutherland I. BCG vaccination of children against leprosy in Uganda: Final results. J Hyg 1981;87:233-48.
[Google Scholar]
|
| 16. |
Tripathy SP. BCG trial in leprosy. Indian J Lepr 1984;56:686-7.
[Google Scholar]
|
| 17. |
Lwin K, Sudaresan T, Gyi MM, Bechelli LM, Tamondong C, Garbajosa PG, et al . BCG vaccination of children against leprosy: Fourteen - year findings of the trial in Burma. Bull WHO 1985;63:1069-78.
[Google Scholar]
|
| 18. |
Scott GC, Russell DA, Boughton CR, Vincin DR. Untreated leprosy: probability for shifts in Ridley-Jopling classification. Development of "flares", or disappearance of clinically apparent disease. Int J Lepr 1976;44:110-22.
[Google Scholar]
|
| 19. |
Chaudhury S, Hazra SK, Saha B, Mazumder B, Biswas PC, Chattopadhya D, et al . An eight-year field trial on anti leprosy vaccines among high-risk household contacts in the Calcutta Metropolis. Int J Lepr 1994;62:389-94.
[Google Scholar]
|
| 20. |
Gupte MD. Field trials of antileprosy vaccines. Indian J Lepr 1998;70:363-7.
[Google Scholar]
|
| 21. |
Gupte MD, Vallishayee RS, Anantharaman DS, Nagaraju B, Sreevatsa, Balasubramanyam S, et al . Comparative leprosy vaccine trial in South India. Indian J Lepr 1998;70:369-88.
[Google Scholar]
|
| 22. |
Fine PE, Ponnighaus JM, Maine N, Clarkson JA, Bliss L. Protective efficacy of BCG against leprosy in Northern Malawi. Lancet 1986;2:499-502.
[Google Scholar]
|
| 23. |
Abel L, Cua VV, ObertiJ, Lap VD, Due LK, Grosset J, et al . Leprosy and BCG in Southern Vietnam (letter). Lancet 1990;335:1536.
[Google Scholar]
|
| 24. |
Muliyil J, Nelson KE, Diamond EL. Effect of BCG on the risk of leprosy in an endemic area: A case-control study. Int J Lepr Other Mycobact Dis 1991;59:229-36.
[Google Scholar]
|
| 25. |
Rodrigues ML, Silva SA, Neto JC, de Andrado AL, Mrtelli CM, Zicker F. Protective effect of intradermal BCG against leprosy: A case-control study in Central Brazil. Int J Lepr 1992;60:335-9.
[Google Scholar]
|
| 26. |
Convit J, Sampson C, Zuniga M, Smith PG, Plata J, Silva J, et al . Immunoprophylactic trial with combined Mycobacterium leprae / BCG vaccine against leprosy: Preliminary results. Lancet 1992;339:446-50.
[Google Scholar]
|
| 27. |
Convit J, Smith PG, Zuniga M, Sampson C, Ulrich M, Plata JA, et al . BCG vaccination protects against leprosy in Venezuela: A case-control study. Int J Lepr Other Mycobact Dis 1993;61:185-91.
[Google Scholar]
|
| 28. |
Barker DM, Nguyen-van-Tam JS, Smith SJ. Protective efficacy of BCG vaccine against leprosy in southern Malawi. Epidemiol Infect 1993;111:21-5.
[Google Scholar]
|
| 29. |
Thuc NV, Abel L, Lap VD, Oberti J, Lagrange PH. Protective effect of BCG against leprosy and its sub-types: A case-control study in Southern Vietnam. Int J Lepr 1994;62:532-8.
[Google Scholar]
|
| 30. |
Boelens JJ, Kroes R, Beers SV, Lever P. Protective effect of BCG against leprosy in South Sulawesi, Indonesia. Int J Lepr Other Mycobact Dis 1995;63:456-7.
[Google Scholar]
|
| 31. |
Lombardi C, Pedrazzani ES, Pedrazzani JC, Filho PF, Zicker F. Protective efficacy of BCG against leprosy in Sao Paulo. Bull Pan Am Health Organ 1996;30:24-30.
[Google Scholar]
|
| 32. |
Bertolli J, Pangi C, Frerichs R, Halloran ME. A case-control study of the effectiveness of BCG vaccine for preventing leprosy in Yangon, Myanmar. Int J Epidemiol 1997;26:888-96.
[Google Scholar]
|
| 33. |
Zodpey SP, Shrikhande SN, Salodkar AD, Maldhure BR, Kulkarni SW. Effectiveness of Bacillus Calmette-Guιrin (BCG) vaccination in the prevention of leprosy: A case-control study in Nagpur, India. Int J Lepr Other Mycobact Dis 1998;66:309-15.
[Google Scholar]
|
| 34. |
Zodpey SP, Bansod BS, Shrikhande SN, Maldhure BR, Kulkarni SW. Protective effect of Bacillus Calmette-Guιrin (BCG) against leprosy: A population based case-control study in Nagpur, India. Lepr Rev 1999;70:287-94.
[Google Scholar]
|
| 35. |
Mohyuddin G. BCG vaccination and leprosy. Indian J Lepr 1992;64:545-6.
[Google Scholar]
|
| 36. |
Lau J. Meta-analyst version 0.99x. New England Medical Center: Boston, MA; 1998.
[Google Scholar]
|
| 37. |
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
[Google Scholar]
|
| 38. |
Prakash O. Antileprosy vaccine: An apprehension. Int J Lepr Other Mycobact Dis 1995;63:572-3.
[Google Scholar]
|
| 39. |
Fine PE. Primary prevention of leprosy. Int J Lepr Other Mycobact Dis 1996;64:S44-9.
[Google Scholar]
|
| 40. |
Fernandez JM. The early reaction induced by lepromin. Int J Lepr Other Mycobact Dis 1940;8:1-14.
[Google Scholar]
|
| 41. |
Springett VH, Sutherland I. Comparison of the efficacy of liquid and freeze-dried strains of BCG vaccine in preventing tuberculosis. Br Med J 1970;4:148-50.
[Google Scholar]
|
| 42. |
Zodpey SP. Application of case-control design and meta-analysis in resolving BCG controversy. 1 st ed. M/s Banarsidas Bhanot Publishers: Jabalpur; 2006. p. 6-140.
[Google Scholar]
|
| 43. |
Fine PE, Rodrigues LC. Modern vaccines: Mycobacterial diseases. Lancet 1990;335:1016-20.
[Google Scholar]
|
| 44. |
Britton WJ. Immunoprophylaxis against leprosy: The case for improved vaccines against leprosy. Int J Lepr Other Mycobact Dis 1996;64:S77-9.
[Google Scholar]
|
| 45. |
Fine PE. Reflections on the elimination of leprosy. Int J Lepr 1992;60:71-80.
[Google Scholar]
|
| 46. |
Fine PE. Leprosy- the epidemiology of a slow bacterium. Epidemiol Rev 1982;4:161-88.
[Google Scholar]
|
| 47. |
Fine PE. The BCG story: Lessons from the past, implications for the future. Rev Infect Dis 1989;11:S353-9.
[Google Scholar]
|
| 48. |
Ponnighaus JM, Fine PE, Sterne JA, Bliss L, Wilson RJ, Malema SS, et al . Incidence rates of leprosy in Karonga District, Northern Malawi: Patterns by age, sex, BCG status and classification. Int J Lepr Other Mycobact Dis 1994;62:10-23.
[Google Scholar]
|
| 49. |
Trial of BCG vaccines in South India for tuberculosis prevention. Indian J Med Res 1979;70:349-63.
[Google Scholar]
|
| 50. |
Trial of BCG vaccines in south India for tuberculosis prevention: First report--Tuberculosis Prevention Trial. Bul World Health Organ 1979;57:819-27.
[Google Scholar]
|
| 51. |
Tuberculosis Prevention Trial, Madras. Indian J Med Res 1980;72:1-74.
[Google Scholar]
|
| 52. |
Fifteen year follow up of trial of BCG vaccines in south India for tuberculosis prevention. Tuberculosis Research Centre (ICMR), Chennai. Indian J Med Res 1999;110:56-69.
[Google Scholar]
|
| 53. |
Fine PE. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet 1995;346:1339-45.
[Google Scholar]
|
Fulltext Views
3,822
PDF downloads
3,718





