Translate this page into:
Pure neuritic leprosy: Current status and relevance
2 CODEWEL Nireekshana Institute, Hyderabad, India
Correspondence Address:
P Narasimha Rao
B-48, Income Tax Colony, Mehdipatnam, Hyderabad - 500 028, Telangana
India
| How to cite this article: Rao P N, Suneetha S. Pure neuritic leprosy: Current status and relevance. Indian J Dermatol Venereol Leprol 2016;82:252-261 |
Abstract
Pure neuritic leprosy has always been an enigma due to its clinical and management ambiguities. Although only the Indian Association of Leprologist's classification recognizes 'pure neuritic leprosy' as a distinct sub group of leprosy, cases nonetheless are reported from various countries of Asia, Africa, South America and Europe, indicating its global relevance. It is important to maintain pure neuritic leprosy as a subgroup as it constitutes a good percentage of leprosy cases reported from India, which contributes to more than half of global leprosy numbers. Unfortunately, a high proportion of these patients present with Grade 2 disability at the time of initial reporting itself due to the early nerve involvement. Although skin lesions are absent by definition, when skin biopsies were performed from the skin along the distribution of the affected nerve, a proportion of patients demonstrated leprosy pathology, revealing sub-clinical skin involvement. In addition on follow-up, skin lesions are noted to develop in up to 20% of pure neuritic leprosy cases, indicating its progression to manifest cutaneous disease. Over the decades, the confirmation of diagnosis of pure neuritic leprosy has been subjective, however, with the arrival and use of high-resolution ultrasonography (HRUS) for nerve imaging, we have a tool not only to objectively measure and record the nerve thickening but also to assess the morphological alterations in the nerve including echo texture, fascicular pattern and vascularity. Management of pure neuritic leprosy requires multidrug therapy along with appropriate dose of systemic corticosteroids, for both acute and silent neuritis. Measures for pain relief, self-care of limbs and physiotherapy are important to prevent as well as manage disabilities in this group of patients.Introduction
The most important consequences of leprosy are the direct result of involvement of peripheral nerves. But for the involvement of peripheral nerves and subsequent deformities, leprosy would have been a simple disease and definitely a disease without stigma.[1] Neuritis in leprosy is usually a sub-acute, demyelinating and non-remitting event involving cutaneous nerves and larger peripheral nerve trunks.
The nerves are immune protected sites due to the inherent blood-nerve barrier. Individual nerve fibers covered by endoneurium are grouped and held together to form nerve fascicles by dense connective tissue constituting the perineurium, along with the blood vessels traversing it. The perineurium forms an effective barrier between the nerve parenchyma and the blood and tissue fluids. During injuries or infections such as leprosy, this barrier can be breached. The pathogenic bacterium Mycobacterium leprae has the unique ability to infect Schwann cells and axons. The presence of Mycobacterium leprae in Schwann cells evokes very little inflammatory response initially, but after a few months to years, the antigenic products of Mycobacterium leprae initiate an immunological recognition and response.
History of Neural Leprosy
Leprosy from ancient times was known to cause skin patches associated with hypo/anesthesia. When the anatomical basis for sensory supply of skin through nerves and nerve trunks was established during the period of medical renaissance, pathologists of the 19th century who performed full-length dissections of peripheral nerves in cadavers of leprosy patients described an ascending neuritis observed by them. Their reports of the macroscopic findings described neuritis of cutaneous nerves originating near the skin lesions and in their subcutaneous trunks, extending proximally for variable distances, ultimately affecting larger nerve trunks and the branches joining them.[2] The extent and type of skin lesions, along with the presence of anesthesia, formed the basis for classification of leprosy in the 19th century. Albert Neisser, in 1903 for the first time mentioned a “neural type of leprosy” and added “lepra nervorum” to the “nodular” and “anesthetic” forms of leprosy already accepted until then.[3] However, “neural leprosy” with only nerve involvement without obvious skin lesions as a separate type of leprosy was first proposed by Wade in 1952. At the International Leprosy Congress, Madrid in 1953, the technical committee included “neuritic leprosy” as one subtype amongst the major groups of leprosy.
The Indian Association of Leprologists (IAL) recognized “neural leprosy” as a distinct type of leprosy and included it in their official six group classification in 1955 and named it “polyneuritic leprosy.” The classification committee noted that the relative prevalence of different forms could vary in different countries and in different parts of the same country. The “neuritic type” was more common in the Indian subcontinent compared to other countries of the world.[3] This classification was followed and used by the National Leprosy Eradication Programme (NLEP) of India. The widely accepted classification for leprosy proposed by Ridley–Jopling in 1966 based on clinical, histological and immunological criteria does not include “neuritic leprosy” in its five-group system. The reason could be the limitations imposed by the criteria they adopted for proposing this classification. The 1982 IAL classification of leprosy persisted with neuritic leprosy as one of the types and named it “pure neuritic type of leprosy”, avoiding the term “poly” which refers to the number of nerves.
Classification of leprosy for therapeutic purposes, first proposed by WHO in 1982, along with the introduction of multidrug therapy, divides leprosy into two categories, paucibacillary and multibacillary, based on skin smear status of the patient and number of skin lesions. No specific mention of “neuritic leprosy” is made in this classification. Leprosy authorities in all countries of the world, including National Leprosy Eradication Programme (NLEP) and International Federation of Anti-Leprosy Associations (ILEP) are following this classification for therapeutic purposes. It is appropriate here to emphasize that some workers consider leprosy to be much more diverse and complex than can be adequately represented in the two-part, “paucibacillary/multibacillary” categorization and that such oversimplification fosters the illusion that this disease is simple and easy to understand or eliminate.[2],[4] Nonetheless, the WHO paucibacillary and multibacillary classification of leprosy is the only one being followed worldwide in field conditions in leprosy endemic countries.
Pure Neuritic Leprosy as an Entity
The original definition of pure neuritic leprosy as approved by IAL in the five-type classification of leprosy of 1982 is as follows: “In this type of leprosy, there are no skin lesions.[5] Larger nerve trunks or their branches are enlarged. There is a sensory loss in the areas of distribution of the nerves. Single or multiple nerves may be involved. Skin smears are negative. Lepromin reaction is generally positive, but sometimes may be doubtful or negative. The histology could be of tuberculoid, borderline or non-specific type.”[5] Despite this elaborate definition, pure neuritic leprosy as a type of leprosy has always been an enigma as clinical and management ambiguities still remain. Nonetheless, there is a worldwide acceptance of pure neuritic leprosy as a distinct type of leprosy. Studies from various countries indicate that this is observed around the world confirming its relevance as a subtype of leprosy.[6],[7],[8],[9] Other terms that have been used as synonyms for pure neuritic leprosy in the literature include neural, neuritic, pure neural, primary neural, primary neuritic, purely neural or poly-neuritic leprosy.[10]
Prevalence
According to Indian studies, pure neuritic leprosy constitutes about 4–18% of leprosy patients.[11] It is reportedly higher in South India comprising up to 18% of new cases. Pure neuritic leprosy is more common in men and it is most common in the 15–30 age group.[12] It is less commonly reported in some parts of the world such as Africa. Although the exact reason for this is not known, it could be epidemiological, as multibacillary leprosy is more common in Africa, unlike in India where paucibacillary leprosy is more common.
Pattern of Nerve Involvement
In pure neuritic leprosy, in general, upper limb nerves are more commonly involved, of which ulnar nerve is the most common. In the lower limb, lateral popliteal nerve is the most common nerve involved, followed by the posterior tibial and sural nerves. However, any nerve trunk or cutaneous nerve can be involved by pure neuritic leprosy, e.g., supraorbital, great auricular, dorsal cutaneous branches of radial, ulnar and superficial peroneal (musculocutaneous) nerve. Selective involvement of the facial nerve branches was also reported.[8] Mononeuritis is the most common presentation of pure neuritic leprosy. However, mononeuritis multiplex which is involvement of multiple unrelated and distant nerve trunks is also observed. In a large study on pure neuritic leprosy from India, it was noted that 26% were mononeuritic while in 39% of cases more than one nerve trunk was involved, either on the same limb or on different limbs.[13] Although “polyneuritic” type of pure neuritic leprosy is reported, some workers prefer to call it “moneuritis multiplex summation.”[12],[14],[15] Symmetrical polyneuritis is uncommon in pure neuritic leprosy and if observed, calls for thorough clinical and bacteriological examination to rule out lepromatous leprosy.
Skin Changes in Pure Neuritic Leprosy
In pure neuritic leprosy, skin along the distribution of the affected nerve is usually hypo-anesthetic or anesthetic, and as a rule, no classical leprosy skin lesions/patches should be present. However, depending on the severity of sensory and autonomic dysfunction, there could be a variable degree of hypo/anhidrosis, xerosis, fissuring and ulcers along its distribution. In addition, if the nerve involved in pure neuritic leprosy is a mixed nerve, there could be motor nerve function impairment, observed as weakness/paralysis of muscles and loss of muscle mass, progressing to deformities.
Nerve Changes in Pure Neuritic Leprosy
By the time patient arrives at the clinic the affected nerve in pure neuritic leprosy is significantly thickened. Sometimes, it can be nodular or beaded and occasionally, in 5–10% of cases, abscess formation is observed. Symptoms of nerve pain can precede clinical diagnosis by a few months to years. Proof of leprosy as a cause of pure neuritic leprosy needs histological evidence which is often sought in affected peripheral nerves. This may be problematic as nerve biopsy is limited by sampling errors, low sensitivity and permanent nerve deficit as functioning nerves often need to be sacrificed.[16]
Some workers observed that nerves of pure neuritic leprosy showed a narrow histological spectrum, ranging from tuberculoid to mid-borderline leprosy only, while others found the entire spectrum from lepromatous changes with acid-fast bacilli to tuberculoid reactions with epithelioid granulomas.[14],[16],[17] In a histopathological study of skin and nerve biopsies in 17 pure neuritic leprosy patients at Karigiri, India, seven (41.2%) were classified in the lepromatous group and 10 (58.8%) in the non-lepromatous group.[18] This study proposed that histological classification would have a bearing on duration of treatment and for their subsequent 'release from treatment' (RFT). In a study at JALMA, Agra, India, where nerve biopsies were taken in 39 out of 108 pure neuritic leprosy cases, although all of the patients were skin smear negative, a significant proportion showed lepromatous histology and nearly two third had a moderate-to-heavy bacterial load within the nerves.[19] In addition, it was observed that there was no relation to clinical parameters such as the number and distribution of affected nerves or the type of immune response.[10]
Histopathology of Apparently Normal Skin and Nasal Mucosa in Pure Neuritic Leprosy
By definition, patients with pure neuritic leprosy should not have clinical skin lesions. However, when skin biopsies were performed in the area of distribution of affected nerves in pure neuritic leprosy, interesting histopathological findings were observed. In a study of 65 patients, skin histopathology from the area of sensory loss revealed non-specific inflammation in the dermis in a majority of patients, with perineural inflammation in a few.[13] However, another study showed histopathological changes of leprosy in the apparently normal looking skin of 32.1% patients with 12.7% of them having either epithelioid or macrophage granulomas.[20] This study concluded that the absence of visible hypopigmented skin lesions in these patients is probably related to the deep location of the granuloma in the dermis, which are, therefore, unable to exercise any direct influence on the melanocytes in the epidermis.
Nasal mucosal involvement was also studied in pure neuritic leprosy.[21] Early changes were seen in the nasal mucosa even before other manifestations. In a study of 39 cases of pure neuritic leprosy, 51% showed specific changes due to leprosy including nerve inflammation, epithelioid granulomas and macrophage collection. Acid-fast bacilli were also demonstrated in the nasal mucosa.
A large study of pure neuritic leprosy patients at Karigiri, South India included 208 patients who had a nerve biopsy, 196 with a skin biopsy and 39 with a nasal mucosal biopsy. Findings in the apparently normal skin and nasal mucosa revealed widespread leprosy pathology even when the disease appeared clinically confined to a few nerves.[22] Nerve biopsy exhibited a spectrum of disease ranging from lepromatous to tuberculoid leprosy with a significant proportion (46%) manifesting as multibacillary leprosy.
Subsequent Development of Skin Lesions in Pure Neuritic Leprosy
There are many reports of patients with pure neuritic leprosy developing leprosy skin patches/lesions during follow-up of months and years, including progression to classical borderline tuberculoid leprosy. In a study of 17 pure neuritic leprosy patients at Karigiri, Southern India, four cases developed skin patches during a follow-up period of 2 years.[18] In a study based in Northern India, 16 pure neuritic leprosy patients developed skin lesions, most within 4 months of diagnosis. The authors concluded that “it appears that pure neuritic leprosy cases with either indeterminate or with advanced multibacillary neural pathology may develop skin lesions.”[23] Others have reported progression to classical borderline tuberculoid leprosy on follow-up.[24] In a study of 182 pure neuritic leprosy patients, 29 patients developed visible skin lesions during follow-up of 2 years. Thirty eight percent of patients developed a single patch and 28% developed two patches. The study concluded that leprosy primarily affects the nerve and that a neuritic phase precedes the development of visible cutaneous lesions.[25]
In a follow-up study of 62 cases of pure neuritic leprosy, 5 out of 20 cases on dapsone monotherapy developed skin lesions after an average duration of 3 months.[26] Of 42 cases treated with rifampicin and dapsone, 3 cases developed skin lesions after a duration of 2–6 months. The diagnosis in cases which developed skin lesions was: borderline lepromatous (1), borderline tuberculoid (4), tuberculoid leprosy (2) and indeterminate (1).
It is well known that new skin lesions can develop during type 1 reactions (reversal reactions) in leprosy patients and this holds good for pure neuritic leprosy as well. In some patients, skin lesions were observed for the first time during type 1 reactions.[27] The appearance of new skin lesions in pure neuritic leprosy supports the hypothesis that leprosy is basically neural in inception and that all other forms emerge from it.[23]
Complications
Nerve abscess
Development of nerve abscess in pure neuritic leprosy is not uncommon. In a 4 year retrospective study, nerve abscesses were noted in 12.5% of cases.[12] Nerve abscesses could be single or multiple. There are reports of multiple abscesses in a single nerve as well as multiple abscesses involving various nerve trunks. A study reported multiple nerve abscesses occurring in supratrochlear, left radial cutaneous, left digital, right superficial peroneal and left saphenous nerves.[28] Multiple nerve abscesses in the same nerve trunk were also reported.[29],[30] Nerve abscesses in leprosy are usually “cold abscesses”, similar to those in tuberculosis and are relatively painless, or have only mild nerve pain.[31] The abscess can be so quiet, without signs of inflammation that they can be mistaken for a soft tissue mass lesion, neuroma or lymphadenitis, especially if they are present on uncommon sites.[32],[33],[34] The size of the abscess does not indicate the extent of resulting nerve function impairment and even a small intraneural abscess can be associated with significant nerve function impairment depending on its location in the nerve.[31]
Segmental necrotizing granulomatous neuritis (SNGN) is a rare condition affecting the nerves in pure neuritic leprosy.[35],[36] It presents as single or multiple nodules of varying sizes along the course of a thickened peripheral nerve. Nerve biopsy shows thickened nerve with multiple foci of caseous necrosis bordered by epithelioid cells and lymphocytes, typical of the entity segmental necrotizing granulomatous neuritis of leprosy. Though very rare, it can be seen in borderline tuberculoid leprosy as well.
Disabilities and deformities
By definition, pure neuritic leprosy patients have sensory deficit along the involved nerve. Unfortunately, most common nerve trunks involved in pure neuritic leprosy such as ulnar, lateral popliteal and posterior tibial nerves provide sensation to hands and feet, thus making sensory impairment in these areas quite common. Consequently, there is early occurrence of WHO grade 1 disability in most cases of pure neuritic leprosy. Unfortunately, progression to grade 2 disability is common, unless recognized and managed promptly.
In a study based at Pune, Central India, pure neuritic leprosy accounted for 179 (4.6%) patients out of the total 3853 patients attending urban leprosy clinics.[10] Of these 179 patients, 87 (48.6%) had deformities at the time of initial presentation. In another study of pure neuritic leprosy from a tertiary care hospital in New Delhi, 50% of patients had various deformities (claw hand, foot drop, trophic changes) at the time of initial presentation.[12]
Clinical Features
As there are no skin lesions as a rule in pure neuritic leprosy, most patients present to leprosy clinics initially either with sudden appearance of numbness with or without ulcers, or with deformity or muscle weakness in a limb. [Table - 1]. Occasionally, they also present with nerve pain (neuropathic pain) associated with 'neuritis.'[6] In a study from Brazil, nerve pain was reported by 42% of patients.[14] Xerosis and fissuring progressing to ulceration are common at the sites of distribution of the affected nerve due to autonomic dysfunction. On examination, the affected nerve, which usually is single in most cases, is often grossly thickened and tender. It should be noted that during reversal reaction (type 1 reaction) in pure neuritic leprosy, the only manifestation would be sudden increase in intensity of neuritis, associated with increased nerve function impairment, as there are no skin lesions to demonstrate the acute signs of inflammation. Perhaps, this is the reason why reversal reactions are not usually recorded or reported frequently in pure neuritic leprosy, unless new skin lesions appear during this phase, which is rare.[27] A study from Oman reported that six patients developed reactional episodes presenting as pain due to severe neuritis, four at the time of presentation and two subsequently.[6] A study from Chandigarh, India, reported reactions in the form of neuritis in 34 (52.3%) of 65 patients; while 30.8% had neuritis at the time of initial presentation, 21.5% developed it during treatment with multidrug therapy.[13] The authors mentioned that no definite figures on reactions are available in other studies on pure neuritic leprosy. The true incidence of type 1 reactions in pure neuritic leprosy is not clear. However, it is known that in the natural history of pure neuritic leprosy there are long periods of mild neuritis interspersed with episodes of severe neuritis indicative of reactions which, in many cases, is the reason for patients seeking medical attention for the first time.[13] In addition, there can be “silent neuritis” or “quiet nerve paralysis” with low-grade inflammation in the nerve leading to incessant nerve damage and deficits. These contribute to the high incidence of disability and deformities in pure neuritic leprosy.[10],[12]
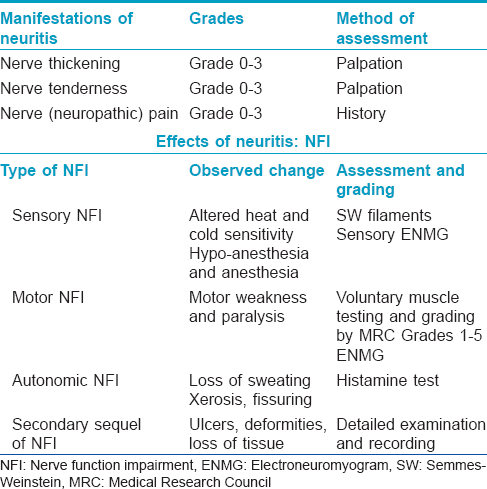
Prognosis
In most cases of pure neuritic leprosy, residual sensory and/or motor and autonomic nerve function impairment of variable degree occurs despite the best treatment. Chronic low-grade inflammation with episodes of severe neuritis leads to destruction of nerve tissue which is replaced by fibrosis. Fibrosis was noted to occur frequently and was reported to occur in 38–79% of all cases.[14],[37] Fibrosis in varying degrees can involve all the three compartments of nerve, the epineurium, perineurium and endoneurium. In a study of 19 untreated cases of pure neuritic leprosy, perineurial and endoneurial fibrosis was observed in 78.9% and 73.6% of cases, respectively.[14] Noordeen, in his field observations from South India, reported that there is a tendency for spontaneous resolution of some cases of pure neuritic leprosy.[38] However, this observation has not been substantiated by other workers. Development of skin lesions and progression of the disease, most commonly to borderline tuberculoid leprosy, are well known, as discussed elsewhere in this article. Nonetheless, with treatment of the disease and of neuritis, inflammation in the nerve usually abates although the nerve thickening could persist for many years.
Investigations in Pure Neuritic Leprosy:[Table - 2]
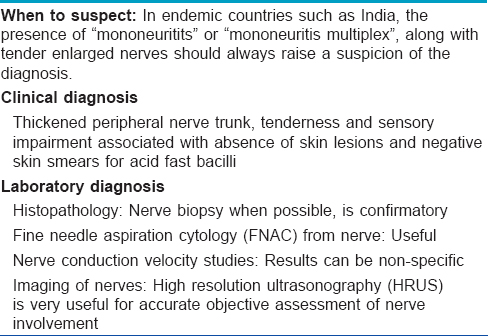
Nerve biopsy in pure neuritic leprosy
The gold standard for confirmation of pure neuritic leprosy is by nerve biopsy. Although any nerve involved would show the pathology, it is prudent to biopsy a branch of a sensory nerve, rather than a mixed nerve or major nerve trunk. The common nerves and sites where nerve biopsy is performed are: superficial sensory radial nerve branch at the wrist, ulnar cutaneous nerve near the ulnar border of base of the hand and sural nerve near the ankle. Proper processing of the nerve biopsy specimen and its staining with hematoxylin and eosin and modified Fite stains is required.[39] The pathologist needs to carefully examine the nerve tissue for changes in its integrity and cellular infiltrates as well as perineural tissues for fibrosis, accumulation of fluid/pus or deposits of calcium. In case nerve biopsy findings are negative or non-specific, the most useful additional tissue samples are from skin with sensory changes and the nasal mucosa. In cases with a high degree of suspicion, multiple small skin punch biopsies (3 mm diameter) will increase the likelihood of picking up specific changes.[16] Nerve biopsy is futile if there is no trained pathologist to read the section.
Fine needle aspiration cytology in pure neuritic leprosy
In leprosy, needle aspiration of an affected nerve for cytological examination can be a valuable tool. Theuvenet et al. first described this procedure which was performed on two cases of pure neuritic leprosy in Nepal in 1993.[40] Fine needle aspiration cytology (FNAC) of nerve was reported to be a valuable tool to confirm leprosy by many studies, both in pure sensory as well as in mixed nerves.[39],[16] It is a safe, less invasive and briefer procedure as compared to nerve biopsy for the diagnosis of pure neuritic leprosy.[41] Aspiration is usually carried out by 22-gauge needle fitted on a 10 ml syringe, inserted along the length of the nerve.[42],[43] Fine needle aspiration cytology from nerves in pure neuritic leprosy yield adequate aspirates for processing as was observed in a study of eight patients.[43]
Cytological smears from the aspirate are stained and read for cellularity, their arrangement and presence of acid-fast bacilli. In addition, polymerase chain reaction to confirm the presence of Mycobacterium leprae can also be performed from the aspirate. Fine needle aspiration cytology proved to be a simple technique to demonstrate inflammation, granulomas and acid-fast bacilli from involved nerves in 18 of the 27 cases suspected to have pure neuritic leprosy.[41] In another study of five suspected cases of pure neuritic leprosy involving common and superficial peroneal, ulnar and median nerve who underwent fine needle aspiration cytology, smears revealed nerve fibers infiltrated by chronic inflammatory cells in all cases, presence of epithelioid cell granulomas and Langhans giant cells in three and acid-fast bacilli in two cases.[42] In another study, fine needle aspiration cytology from eight cases of pure neuritic leprosy revealed epithelioid cell granulomas in three cases and acid-fast bacilli (bacterial index 1+) in one case. This study concluded that fine needle aspiration cytology of the nerve yields diagnostic aspirates in leprosy comparable with nerve pathology observed on nerve biopsy.[43]
The only limitation of this technique is that a negative aspirate does not rule out leprosy. In general, fine needle aspiration cytology, being a simpler, quicker and less invasive technique can be attempted on the nerve before deciding on a nerve biopsy. This would be particularly useful when pure neuritic leprosy is suspected.[43]
Polymerase chain reaction (PCR) has been found to be especially valuable in diagnosing leprosy in difficult situations such as pure neural cases, paucibacillary disease and patients with atypical clinical presentation and histopathological features compatible with leprosy.[44] In leprosy studies, quantitative PCR assays amplifying different Mycobacterium leprae gene targets, sodA, 16S ribosomal RNA, RLEP and Ag 85B are being tried.[45] When PCR amplification of the Mycobacterium leprae-specific 16S ribosomal RNA was performed, the detection rate in multibacillary and paucibacillary patients was 100% and 50%, respectively while the specificity was 100 percent.[46] Some workers have performed both cytological examination as well PCR on the nerve aspirates. In a study performed in Eastern India, out of the 13 cases where FNAC was done, acid-fast bacilli were found in 5 (38.4%) cases; however, in 11 (84.6%) of these aspirates, PCR confirmed Mycobacterium leprae.[47]
Nerve Conduction Studies
There are a number of studies on motor and sensory nerve conduction which have shown that marked slowing of conduction can occur in leprosy-affected nerves. The electroneuromyographic pattern of leprosy neuropathy described in the literature is the impairment of conduction of nerve impulse and decreased amplitude of sensory-motor potentials.[9] Along with reduction in nerve conduction velocity, changes in latency were also observed. When sensory conduction velocity was compared between leprosy patients and normal subjects, slowing was shown in all nerves, with no difference between tuberculoid and lepromatous patients. In addition, a significant slowing of nerve conduction has also been reported in clinically normal nerves in leprosy. However, studies have reported on the absence of correlation between neurological symptoms and electroneurographic studies in leprosy patients.[48] A study combining nerve palpation with either Semmes–Weinstein monofilament testing or voluntary muscle testing, increased the detection rate of nerve abnormalities by 2-fold, and was comparable to the detection rate by nerve conduction studies.[49]
Imaging of Nerves
The hallmarks of leprosy are nerve enlargement and inflammation. Magnetic resonance imaging (MRI) and high-resolution ultrasonography (HRUS) can be used for imaging of nerves. Developments in ultrasonography have made available transducers with high resonance frequency (15–20 MHz) which have made it very effective to visualize nerves. Although MRI can be performed to image the integrity of peripheral nerve trunks, high-resolution ultrasonography is much more efficient, user-friendly and economical and therefore, more popular and widely applied.[50] Additional features such as compound imaging and panorama view make high-resolution ultrasonography a superior modality for imaging of nerves. High-resolution ultrasonography demonstrates nerve enlargement, even if subclinical. Inflammation can be detected by colour doppler study of involved nerves which show increased blood flow signals of endoneural and perineural vessels.[51] In a study of ulnar nerves in 21 leprosy patients by high-resolution ultrasonography, a positive correlation was observed between the presence of motor weakness, sonographic thickening of the ulnar nerve and slowing of motor conduction.[52]
Importance of Imaging of Nerves in Pure Neuritic Leprosy
High-resolution ultrasonography is of proven value in visualizing structural changes of major nerves trunks in all types of leprosy. It is of specific use in pure neuritic leprosy as it objectively confirms nerve thickening in the absence of other cutaneous signs of leprosy. Peripheral nerve high-resolution ultrasonography provides information on the exact location of nerve enlargement and morphological alterations in the nerve including echo texture, fascicular pattern and vascularity.[51]
This information brings a new dimension to the diagnosis of pure neuritic leprosy and the early recognition of leprosy neuritis, especially during reactional phases of the disease.[53] The increased blood flow and vascularity observed on ultrasound were correlated with edema and vascularity histologically showing that ultrasound could be a non-invasive tool to recognize neuritis and to indicate the need for corticosteroid therapy to prevent permanent nerve damage associated with reactions.[54] In addition, it is of great value in clinical situations where thickening of nerves is equivocal, when there is motor/sensory deficit with apparently normal nerves or to confirm fibrosis, abscess and calcification in the nerve trunk.
Management of Pure Neuritic Leprosy
Is pure neuritic leprosy paucibacillary or multibacillary for therapeutic purposes?
The only classification which the WHO advocates for leprosy from the year 1982 is paucibacillary/multibacillary for therapeutic purposes, based on skin smear status and number of skin lesions. There are no guidelines from the WHO about the classification of pure neuritic leprosy depending on number of nerves involved and, therefore, its treatment.[55] However, many consider that pure neuritic leprosy belongs to the paucibacillary group since all of them are acid-fast bacilli negative on skin smears by definition and are mostly lepromin positive. This was also the recommendation made by the National Leprosy Eradication Programme (NLEP) of India in 1987, which grouped pure neuritic leprosy within paucibacillary leprosy for therapeutic purposes.[56]
Although skin smears are always negative, nerve biopsy in several cases of pure neuritic leprosy has revealed features of borderline or even lepromatous leprosy along with acid-fast bacilli. However, it would be impractical to perform nerve biopsy routinely in patients of pure neuritic leprosy to classify the disease for treatment purpose as most common nerves affected are mixed nerves. Hence, the number of nerve trunks involved is taken as a clinical criterion for determining the multidrug therapy regimen (paucibacillary/multibacillary). According to present NLEP guidelines in India, when one nerve trunk is involved in leprosy it is considered as paucibacillary, and when more than one nerve trunk is involved, it is considered as multibacillary for therapeutic purposes.[57] This definition for therapy is being applied at present, both to pure neuritic as well as paucibacillary leprosy in India. As treatment guidelines for pure neuritic leprosy as a distinct type of leprosy are not in place at present, well-planned studies are required to formulate the right multidrug therapy regimen taking into account aspects such as the number of involved nerve trunks, cutaneous nerve twigs and anatomical distribution.[55] In case of ambiguity regarding number of nerves involved, it is advisable to treat it as multibacillary leprosy. High-resolution ultrasonography of nerves is of immense value in such cases.
Management of neuritis
In pure neuritic leprosy, there is always some neuritis associated with the disease. In addition, episodes of severe neuritis often occur. It is imperative that episodes of severe neuritis be managed with appropriate dosage and duration of corticosteroid therapy.[13] Unfortunately, there are no studies or guidelines on how to manage either chronic simmering or acute neuritis in such cases. It is only logical that the principles of treatment and corticosteroid dosages for episodes of acute neuritis should be similar to those recommended for the management of neuritis of type 1 reaction. Even during periods of quiescence, clinicians need to be watchful for “silent neuropathy” or “quiet nerve paralysis” in which nerve trunks get quietly paralyzed in a proportion of leprosy patients without going through a stage of acute or subacute neuritis resulting in insidious increase in nerve function deficit.[58] Once identified, silent neuropathy needs to be managed with tapering doses of corticosteroids over months.[58] In addition, neuropathic pain is known to occur in pure neuritic leprosy which should be managed with appropriate drug therapy such as tricyclic antidepressants and anticonvulsants.[1],[59] When new lesions start appearing in due course, as can happen in some cases of pure neuritic leprosy, a change in type of multidrug therapy may need to be considered.
As sensory impairment of hands and feet is common, teaching self-care of limbs to prevent further disability and deformity is of utmost importance. Furthermore, as pure neuritic leprosy cases are known to present more frequently with deformities, physiotherapy and other corrective measures must be employed early. These measures need to be continued even after antileprosy treatment is stopped, often for long periods of time in most patients.
Importance and relevance of pure neuritic leprosy as a distinct leprosy group
If skin patches and presence of acid-fast bacilli were the only criteria used for diagnosis of leprosy, it is obvious that all the cases of pure neuritic leprosy which constitute a good percentage of all leprosy patients would be missed. Hence, retaining pure neuritic leprosy as a distinct type of leprosy is important for leprosy programs. The fact that skin lesions are noted to occur in up to 20% of pure neuritic leprosy over months and years of observation indicates that this is a form of leprosy with initial pronounced nerve trunk involvement followed by cutaneous manifestations in a good proportion of cases.[18],[20],[23] In other words, categorizing pure neuritic leprosy as a group and clinically diagnosing these cases early equates to identifying leprosy before the skin is involved. Such early identification of pure neuritic leprosy and its treatment is of great benefit to the patient as it limits the extent and progression of nerve damage and resulting disability.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Rao PN, Suneetha S. Neuritis-definition, clinico-pathological manifestations and proforma to record nerve impairment in leprosy. In: Kar HK, Kumar B, editors. IAL Textbook of Leprosy. 1st ed. New Delhi: Jeypee Brothers Medical Publishers (P) Ltd.; 2010. p. 253-68.
[Google Scholar]
|
| 2. |
Scollard DM. The biology of nerve injury in leprosy. Lepr Rev 2008;79:242-53.
[Google Scholar]
|
| 3. |
Prasad PV, editor. All about Leprosy. New Delhi: Jeypee Brothers Medical Publishers (P) Ltd.; 2005.
[Google Scholar]
|
| 4. |
Scollard DM. Classification of leprosy: A full color spectrum, or black and white? Int J Lepr Other Mycobact Dis 2004;72:166-8.
[Google Scholar]
|
| 5. |
Cinical histopathological and immunological features of the five type classification approved by the Indian association of leprologists. Lepr India 1982;54:22-5.
[Google Scholar]
|
| 6. |
Al Suwaid AR, Venkatram MN, Banodkar DD. Study of pure neuritic leprosy in Oman. Gulf J Dermatol 1994;1:25-7.
[Google Scholar]
|
| 7. |
Lockwood DN, Reid AJ. The diagnosis of leprosy is delayed in the United Kingdom. QJM 2001;94:207-12.
[Google Scholar]
|
| 8. |
Mafoyane NA, Jacyk WK, Lotz BP. Primary neuritic leprosy in a black South African. Lepr Rev 1992;63:277-81.
[Google Scholar]
|
| 9. |
Skacel M, Antunes SL, Rodrigues MM, Nery JA, Valentim VD, Morais RP, et al. The diagnosis of leprosy among patients with symptoms of peripheral neuropathy without cutaneous lesions: A follow-up study. Arq Neuropsiquiatr 2000;58:800-7.
[Google Scholar]
|
| 10. |
Mahajan PM, Jogaikar DG, Mehta JM. A study of pure neuritic leprosy: Clinical experience. Indian J Lepr 1996;68:137-41.
[Google Scholar]
|
| 11. |
Sharma VK, Malhotra AK. Leprosy: Classification and clinical aspects. In: Valia RG, Valia AR, editors. IADVL Text Book of Dermatology. 3rd ed. Mumbai: Bhalani Publishing House; 2008. p. 2032-69.
[Google Scholar]
|
| 12. |
Mendiratta V, Khan A, Jain A. Primary neuritic leprosy: A reappraisal at a tertiary care hospital. Indian J Lepr 2006;78:261-7.
[Google Scholar]
|
| 13. |
Kumar B, Kaur I, Dogra S, Kumaran MS. Pure neuritic leprosy in India: An appraisal. Int J Lepr Other Mycobact Dis 2004;72:284-90.
[Google Scholar]
|
| 14. |
Jardim MR, Antunes SL, Santos AR, Nascimento OJ, Nery JA, Sales AM, et al. Criteria for diagnosis of pure neural leprosy. J Neurol 2003;250:806-9.
[Google Scholar]
|
| 15. |
Nascimento OJ. Leprosy neuropathy: Clinical presentations. Arq Neuropsiquiatr 2013;71:661-6.
[Google Scholar]
|
| 16. |
Smith EW. Diagnosis of pure neuritic leprosy. Neurol J Southeast Asia 2002;7:61-3.
[Google Scholar]
|
| 17. |
Uplekar MW, Antia NH. Clinical and histopathological observations on pure neuritic leprosy. Indian J Lepr 1986;58:513-21.
[Google Scholar]
|
| 18. |
Pannikar VK, Arunthathi S, Chacko CJ, Fritschi EP. A clinico-pathological study of primary neuritic leprosy. Lepr India 1983;55:212-21.
[Google Scholar]
|
| 19. |
Kaur G, Girdhar BK, Girdhar A, Malaviya GN, Mukherjee A, Sengupta U, et al. A clinical, immunological, and histological study of neuritic leprosy patients. Int J Lepr Other Mycobact Dis 1991;59:385-91.
[Google Scholar]
|
| 20. |
Suneetha S, Arunthathi S, Chandi S, Kurian N, Chacko CJ. Histological studies in primary neuritic leprosy: Changes in the apparently normal skin. Lepr Rev 1998;69:351-7.
[Google Scholar]
|
| 21. |
Suneetha S, Arunthathi S, Job A, Date A, Kurian N, Chacko CJ. Histological studies in primary neuritic leprosy: Changes in the nasal mucosa. Lepr Rev 1998;69:358-66.
[Google Scholar]
|
| 22. |
Suneetha S, Arunthathi S, Kurian N, Chacko CJ. Histological changes in the nerve, skin and nasal mucosa of patients with primary neuritic leprosy. Acta Leprol 2000-2001;12:11-8.
[Google Scholar]
|
| 23. |
Mishra B, Mukherjee A, Girdhar A, Husain S, Malaviya GN, Girdhar BK. Neuritic leprosy: Further progression and significance. Acta Leprol 1995;9:187-94.
[Google Scholar]
|
| 24. |
Porichha D, Mahapatra DC. Borderline tuberculoid leprosy developing in a pure neuritic case. Indian J Lepr 1991;63:235-7.
[Google Scholar]
|
| 25. |
Suneetha S, Sigamoni A, Kurian N, Chacko CJ. The development of cutaneous lesions during follow-up of patients with primary neuritic leprosy. Int J Dermatol 2005;44:224-9.
[Google Scholar]
|
| 26. |
Talwar S, Jha PK, Tiwari VD. Neuritic leprosy: Epidemiology and therapeutic responsiveness. Lepr Rev 1992;63:263-8.
[Google Scholar]
|
| 27. |
Guilloton L, Drouet A, Combemale P, Cruel T, Dupin M, Ribot C. Neuritic leprosy disclosed by reversal reaction. Rev Neurol (Paris) 2002;158:84-6.
[Google Scholar]
|
| 28. |
Laxmisha C, Thappa DM, Kumar MS, Joseph LC, Jayanthi S. Pure neural leprosy presenting with multiple nerve abscesses. Indian J Lepr 2004;76:343-50.
[Google Scholar]
|
| 29. |
Rai D, Malhotra HS, Garg RK, Goel MM, Malhotra KP, Kumar V, et al. Nerve abscess in primary neuritic leprosy. Lepr Rev 2013;84:136-40.
[Google Scholar]
|
| 30. |
Ankad BS, Jawalgi A, Dombale VD, Telkar S. Multiple nerve abscesses on cutaneous radial nerve – a case of pure neural leprosy. Indian J Lepr 2013;85:79-81.
[Google Scholar]
|
| 31. |
Srinivasan H. Management of Nerve Abscess. Leprosy Mailing List Blog. Available from: http://www.leprosymailinglist.blogspot.in/2008/08/management-of-nerve-abscess.html. [Last accessed on 2015 Jul 26].
[Google Scholar]
|
| 32. |
Omar AE, Hussein MR. Clinically unsuspected neuritic leprosy with caseation necrosis. Ultrastruct Pathol 2012;36:377-80.
[Google Scholar]
|
| 33. |
Siddaraju N, Sistla SC, Singh N, Muniraj F, Chahwala Q, Basu D, et al. Pure neuritic leprosy with nerve abscess presenting as a cystic, soft tissue mass: Report of a case diagnosed by fine needle aspiration cytology. Diagn Cytopathol 2009;37:355-8.
[Google Scholar]
|
| 34. |
Chugh S, Barman KD, Goel K, Garg VK. Leprosy nerve abscess in Indian male, misdiagnosed as tuberculous lymphadenitis and neuroma. Lepr Rev 2013;84:158-60.
[Google Scholar]
|
| 35. |
Chandi SM, Chacko CJ, Fritschi EP, Job CK. Segmental necrotizing granulomatous neuritis of leprosy. Int J Lepr Other Mycobact Dis 1980;48:41-7.
[Google Scholar]
|
| 36. |
Jayalakshmy PS, Prasad PH, Kamala VV, Aswathy R, Pratap P. “Segmental necrotizing granulomatous neuritis”: A rare manifestation of hansen disease-report of 2 cases. Case Rep Dermatol Med 2012;2012:758093.
[Google Scholar]
|
| 37. |
de Freitas MR, Nascimento OJ, Drago MJ, de Freitas AR, Hahn MD. Ulnar nerve palsy in leprosy without skin changes: Biopsy of the superficial branch of the ulnar nerve in the hand. Arq Neuropsiquiatr 1998;56:585-94.
[Google Scholar]
|
| 38. |
Noordeen SK. Epidemiology of (Poly) neuritic type of leprosy. Lepr India 1972;44:90-6.
[Google Scholar]
|
| 39. |
Suneetha S. Histopathological techniques. In: Revised Hand-book of Medical Laboratory Technology. 2nd ed. Karigiri: Laboratory Training Committee of CMAI; 1993. p. 508-41.
[Google Scholar]
|
| 40. |
Theuvenet WJ, Miyazaki N, Roche P, Shrestha I. Cytological needle aspiration of the nerve for the diagnosis of pure neural leprosy. Int J Lepr Other Mycobact Dis 1993;61:597-9.
[Google Scholar]
|
| 41. |
Jayaseelan E, Shariff S, Rout P. Cytodiagnosis of primary neuritic leprosy. Int J Lepr Other Mycobact Dis 1999;67:429-34.
[Google Scholar]
|
| 42. |
Kumar B, Pradhan A. Fine needle aspiration cytology in diagnosis of pure neuritic leprosy. Patholog Res Int 2011;2011:158712.
[Google Scholar]
|
| 43. |
Vijaikumar M, D'Souza M, Kumar S, Badhe B. Fine needle aspiration cytology (FNAC) of nerves in leprosy. Lepr Rev 2001;72:171-8.
[Google Scholar]
|
| 44. |
Martinez AN, Talhari C, Moraes MO, Talhari S. PCR-based techniques for leprosy diagnosis: From the laboratory to the clinic. PLoS Negl Trop Dis 2014;8:e2655.
[Google Scholar]
|
| 45. |
Martinez AN, Ribeiro-Alves M, Sarno EN, Moraes MO. Evaluation of qPCR-based assays for leprosy diagnosis directly in clinical specimens. PLoS Negl Trop Dis 2011;5:e1354.
[Google Scholar]
|
| 46. |
Bang PD, Suzuki K, Phuong le T, Chu TM, Ishii N, Khang TH. Evaluation of polymerase chain reaction-based detection of Mycobacterium leprae for the diagnosis of leprosy. J Dermatol 2009;36:269-76.
[Google Scholar]
|
| 47. |
Reja AH, De A, Biswas S, Chattopadhyay A, Chatterjee G, Bhattacharya B, et al. Use of fine needle aspirate from peripheral nerves of pure-neural leprosy for cytology and PCR to confirm the diagnosis: A pilot study. Indian J Dermatol Venereol Leprol 2013;79:789-94.
[Google Scholar]
|
| 48. |
Tzourio C, Said G, Millan J. Asymptomatic nerve hypertrophy in lepromatous leprosy: A clinical, electrophysiological and morphological study. J Neurol 1992;239:367-74.
[Google Scholar]
|
| 49. |
Khambati FA, Shetty VP, Ghate SD, Capadia GD. Sensitivity and specificity of nerve palpation, monofilament testing and voluntary muscle testing in detecting peripheral nerve abnormality, using nerve conduction studies as gold standard; a study in 357 patients. Lepr Rev 2009;80:34-50.
[Google Scholar]
|
| 50. |
Rao PN, Jain S. Newer management options in leprosy. Indian J Dermatol 2013;58:6-11.
[Google Scholar]
|
| 51. |
Jain S, Visser LH, Praveen TL, Rao PN, Surekha T, Ellanti R, et al. High-resolution sonography: A new technique to detect nerve damage in leprosy. PLoS Negl Trop Dis 2009;3:e498.
[Google Scholar]
|
| 52. |
Bathala L, Kumar K, Pathapati R, Jain S, Visser LH. Ulnar neuropathy in hansen disease: Clinical, high-resolution ultrasound and electrophysiologic correlations. J Clin Neurophysiol 2012;29:190-3.
[Google Scholar]
|
| 53. |
Elias J Jr., Nogueira-Barbosa MH, Feltrin LT, Furini RB, Foss NT, Marques W Jr., et al. Role of ulnar nerve sonography in leprosy neuropathy with electrophysiologic correlation. J Ultrasound Med 2009;28:1201-9.
[Google Scholar]
|
| 54. |
Jain S, Visser LH, Yerasu MR, Raju R, Meena AK, Lokesh B, et al. Use of high resolution ultrasonography as an additional tool in the diagnosis of primary neuritic leprosy: A case report. Lepr Rev 2013;84:161-5.
[Google Scholar]
|
| 55. |
Handa S, Dogra S. Hot topics in leprosy. Lepr Rev 2003;74:87-8.
[Google Scholar]
|
| 56. |
Leprosy: Guidelines on Case Detection, Treatment Follow-up and Reporting, NLEP 1987. Leprosy Division, Directorate General of Health Services, Nirman Bhavan, New Delhi; 1987.
[Google Scholar]
|
| 57. |
NLEP Training Manual for Medical Officer, Central Leprosy Division, DGHS, Ministry of heath and family welfare (GOI), Nirman Bhavan, New Delhi. 2013
[Google Scholar]
|
| 58. |
Srinivasan H, Rao KS, Shanmugam N. Steroid therapy in recent “quiet nerve paralysis” in leprosy. Report of a study of twenty-five patients. Lepr India 1982;54:412-9.
[Google Scholar]
|
| 59. |
Haanpää M, Lockwood DN, Hietaharju A. Neuropathic pain in leprosy. Lepr Rev 2004;75:7-18.
[Google Scholar]
|
Fulltext Views
24,382
PDF downloads
5,274





