Translate this page into:
Quality of life in acne vulgaris: Relationship to clinical severity and demographic data
2 Department of Community Medicine, Dr. D.Y. Patil Medical College and Hospital, Pimpri, Pune, Maharashtra, India
Correspondence Address:
Aayush Gupta
B-402, The Metropolitan, Near Darshan Hall, Chinchwad, Pune - 411 033, Maharashtra
India
| How to cite this article: Gupta A, Sharma YK, Dash KN, Chaudhari ND, Jethani S. Quality of life in acne vulgaris: Relationship to clinical severity and demographic data. Indian J Dermatol Venereol Leprol 2016;82:292-297 |
Abstract
Background: Acne vulgaris is known to impair many aspects of quality of life. However, the correlation of this impairment with clinical severity remains equivocal despite various school, community and hospital-based studies. Aim: A hospital-based study was undertaken to measure the impairment of quality of life of patients of acne vulgaris and correlate it with the severity of lesions. Methods: This was a cross-sectional, questionnaire-based study in a cohort of 100 patients of acne vulgaris attending the outpatient department of our referral hospital. A physician measured the severity of lesions using the global acne grading system, and patients assessed quality of life by completing a questionnaire (Cardiff acne disability index). A correlation of these two was done; some additional correlations were brought out through demographic data collected from the patients. Results: There was no correlation between the severity of acne vulgaris and an impaired quality of life. Patients who consumed alcohol and/or smoked cigarettes were found to have an impaired quality of life. While the severity of acne progressively lessened in older patients, the impact on quality of life increased. Limitations: The sample size was small and there was a lack of guaranteed reliability on the self-reported quality of life. Conclusion: The severity of acne vulgaris does not correlate with impairment in quality of life.Introduction
Acne vulgaris is the commonest skin condition affecting more than 80% of individuals at some stage of their life.[1],[2] In many patients, rather than being a self-limiting condition of adolescence, acne vulgaris acquires all the characteristics of a chronic disorder as defined by the World Health Organization, viz a prolonged course, a pattern of recurrence or relapse, manifesting as acute outbreaks or slow onset, and a psychological and social impact on the individual's quality of life.[3]
Though impairment in the quality of life of patients of acne vulgaris is well established, its direct correlation with clinical severity has not been established.[4] The profound suffering of these patients in terms of social, vocational and academic performance makes them seek professional help more often for non-cutaneous manifestations e.g., poor body image, anxiety, depression, anger, frustration, diminished self-esteem and confidence, social isolation and restriction of activities.[5],[6],[7],[8] We undertook this study to delineate the factors contributing to the impairment of quality of life and to ascertain their correlation, if any, with the clinical severity of acne vulgaris.
Methods
This cross-sectional, questionnaire-based study was carried out after receiving ethical clearance from our Institute, on a cohort of 100 patients of acne vulgaris attending the outpatient department of our referral tertiary care hospital. After obtaining informed consent, each patient was asked to fill in, without any time limit, their demographic details, and the Hindi version of the Cardiff acne disability index questionnaire.[9] This index is a well-accepted five-question scale designed to assess the disability caused by acne. Its Hindi translation and validation done by our department has since been accepted by the original authors. The first two questions in this index address the psychological, social and sexual consequences of acne in general; question three asks about the avoidance of exposure (e.g., hesitation to wear swimming costumes) by those with acne of the chest or back; question four attempts to determine the patient's psychological state and question five, the patient's (subjective) assessment of current acne severity. The response to each question is scored from 0 to 3, a higher score indicating greater disability.[10] The clinical severity of acne was assessed using the global acne grading system score which calculates global score by adding the individual scores of six locations, five on the face (forehead, right and left cheek, nose and chin) and the sixth, a combined one for chest and upper back, each location having already been assigned a fixed score based on the surface area, distribution and density of pilosebaceous units. A score of 1–18 is considered as mild; 19–30 as moderate; 31–38 as severe and more than 39 as very severe.[11]
Quantitative variables were described using percentages, ranges, means and standard deviations. Student's independent t-test, analysis of variance test and Spearmen's correlation analysis were performed using the statistical package for the social sciences version 20 (SPSS Inc., Chicago, IL, USA) as appropriate. A two-tailed probability value of 0.05 or less was considered as statistically significant.
Results
Demographic data
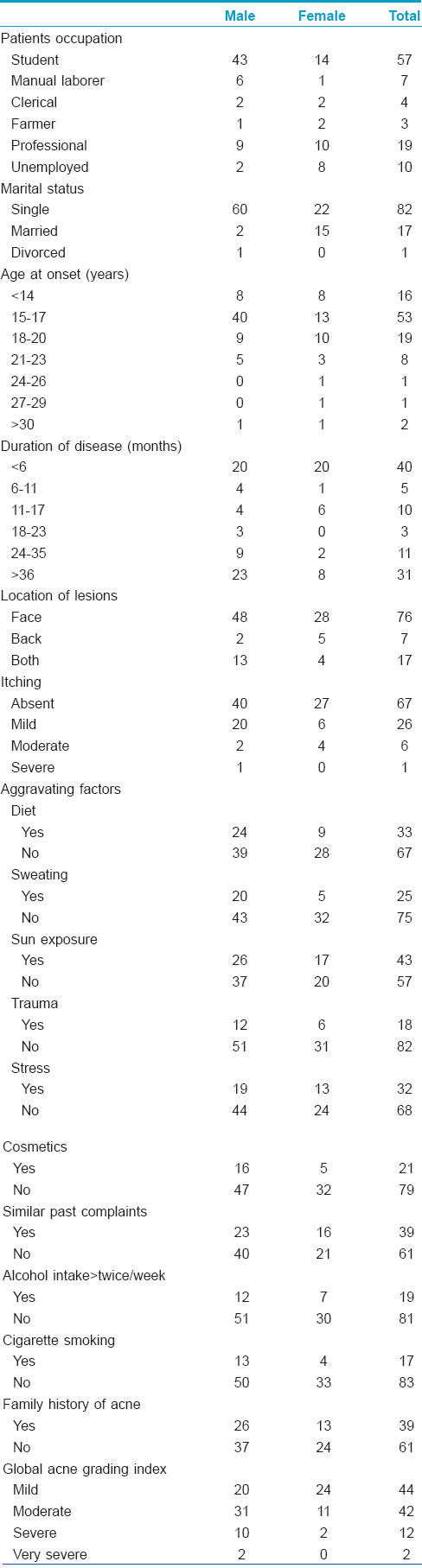
The study population consisted of 100 patients (63 men, 37 women) between the ages of 14–45 (mean: 22.49 ± 5.381) years. Students (57), professionals (19) and housewives (10) formed the majority. Eighty-two of them were single; 17 married and one was divorced. The majority (53%) first noticed acne lesions between 15 and 17 years of age; 19% between 18 and 20 years and less than16%, under 14 years of age. Forty percent consulted our department within 6 months of onset while 31% did so after 3 years; 48% presented during their first spell of acne; the remaining, after having had treatment earlier. Interestingly, 47% gave a history of having undergone a period of stress within the previous month. In over three-fourths (76%), lesions were only on the face; in 17%, the trunk was also affected in addition to their face while the lesions were only on the trunk in the remaining 13%. The majority (67%) of the patients reported no itching related to acne lesions. Sunlight was the principal aggravating factor in 45% of either sex. In females, pre- and post-menstrual flares (75.7 and 5.4%) were common. Acne worsened during the summer in 26% cases. Nineteen percent of patients consumed alcohol more than twice weekly; 17% smoked cigarettes. A family history of acne was present in first-degree relatives of 39% of our study patients. Body mass index ranged between 17.04 to 29.41 (mean: 20.92 ± 2.72); only seven patients had a body mass index of greater than 25 [Table - 1].
Cardiff acne disability index
The overall mean Cardiff acne disability index was 6.09 (±3.153, range 0–15), higher in males (6.33 ± 0.385) than females (5.68 ± 0.544). Question 5 had the highest (1.67) mean score; question 3, the lowest (0.64). There was a positive correlation (0.230, P = 0.05) of the patient's age with the Cardiff acne disability index score. Sex, occupation, marital status, age of onset, duration, location of lesions, severity of itching and aggravating factors had no correlation with the Cardiff acne disability index score. Patients who drank alcohol more than twice a week had a significantly higher index (7.68 ± 2.23, P = 0.014) than those who did not consume alcohol at all (5.72 ± 3.23). Cigarette smokers also had a significantly higher score (7.88 ± 2.34, P = 0.09) than non-smokers (5.72 ± 3.18). Patients (11) who took alcohol as well as smoked cigarettes had the highest score (7.91 ± 2.548), much more than those who did neither (5.87 ± 3.16, P = 0.042). Patients whose fathers had also suffered from acne of any grade had a significantly higher score (8.25 ± 2.31, P = 0.043) as compared to those patients who had no such family history (5.90 ± 3.15). There was no difference in the impairment of the quality of life between patients who had previously taken treatment for acne and those who had not.
Global acne grading system
The overall mean global acne grading system score was 21.43 (±6.73, range 10–40); the two highest individual scores were for the cheeks: right (5.40) and left (5.24). Mild, moderate, severe and very severe acne were present in 44%, 42%, 12% and 2% of the patients, respectively. Global acne grading system score was significantly higher in males (23.29 ± 6.576) than in females (18.27 ± 5.805) (P = 0.01). There was a significant negative correlation between the age and the severity score (−0.245, P = 0.014) of the patients. Occupation, marital status, age of onset, duration of disease, decreased sleep, alcohol intake, smoking, hypertension or diabetes mellitus, previous treatment, body mass index, itching and aggravating factors of sweating, sun exposure, trauma, stress and menstruation revealed no correlation with the global acne grading system score in our study. However, people who reported diet to be an aggravating factor had a significantly higher global acne grading system score (24.89 ± 7.219, P = 0.01) than those who did not (19.73 ± 5.809). Global acne grading system score was also significantly higher in patients without any family history of acne (22.56 ± 6.757, P = 0.035) than in those with such a history (19.67 ± 6.372).
Quality of life vis-a-vis clinical severity
There was no correlation between the Cardiff acne disability index and global acne grading system scores (r = 0.83). There was a weak positive correlation between a higher severity score of the left cheek and Cardiff acne disability index (r = 0.224, P = 0.025) score.
Discussion
The relationship between dermatologic disorders and the mind has been of interest since 1891 when Brocq and Jacquet first described “neurodermatitis.”[12] Prevalence estimates for emotional disorders among dermatological patients range from 25% for outpatients to 45% for inpatients suggesting an important, albeit, complex relationship between skin diseases and the personality and psycho-emotional functioning of the patient.[13],[14] General practitioners as well as dermatologists have been reported, to have poor comprehension of the psychological implications of skin diseases, to be insensitive to the emotional suffering of patients and to trivialize the disease.[15] Patients with acne have been reported to have functional and emotional effects comparable to those in patients having eczema or psoriasis and equivalent or greater levels of social, psychological and emotional problems as seen in patients with chronic disabling medical or surgical diseases (asthma, epilepsy, diabetes, back pain or arthritis). Better understanding of factors affecting acne vulgaris may, therefore, serve to identify patients needing special attention.[8],[16]
Our study reiterates the significant impairment in quality of life in patients of acne vulgaris reported in earlier studies. However, in contrast to the female preponderance seen in previous studies,[8],[17],[18],[19] Cardiff acne disability index score was higher in males in our study. Stern, in a large retrospective analysis of the United States federal outpatient records for the period of 1980–1997, found 80% more visits to dermatologists by females.[20]
Early (40%, within 6 months) or delayed (31%, after 3 years) reporting after the onset of acne by our study population affected neither their quality of life nor clinical severity.
The total mean Cardiff acne disability index score in our study was 6.09 which was higher than that in Serbian, Scottish and Egyptian studies.[21],[22] Higher grades of acne in our study population could be explained by this being an institutional rather than a community-based study. A higher Cardiff acne disability index score has also been reported in another hospital-based Persian study.[23]
On analysing responses to individual questions in the Cardiff acne disability index, the highest score in our study was to the question on subjective scoring of acne severity which underscores the subjective differences in the psyche of individual patients. The lowest score was to the third question about the impact of acne on the chest or back during the use of public changing rooms. This was probably because there were mainly men in our study and the women who were included did not wear backless dresses/bikinis.
Progressive worsening of acne-related quality of life with increasing age recorded in our study, as also in an earlier one, may probably be due to unknown factor(s) causing increased vulnerability of the aged.[8] A significantly higher impact on the quality of life of patients with substance abuse (smoking or drinking more than twice a week) necessitates cultivation by the treating physician of extra sensitivity toward these patients. The significance of higher Cardiff acne disability index in children whose fathers had a history of acne needs unraveling by future studies.
The mean global acne grading system score in our study was higher than in most of the previous studies, probably because it was conducted at a referral institution. It had a significant negative correlation with age, i.e. while the severity of acne reduced with increasing age, the impairment in quality of life became more.[17],[23],[24] The score was also higher in patients who either reported having diet as an aggravating factor or had diminished appetite. The apparently surprising higher global acne grading system score in patients without a family history of acne vulgaris, too, necessitates further validation. However, no significant relationship was noticed between a history of previous treatment and a lower global acne grading system score or between a higher body mass index and higher global acne grading system score.
Quality of life based on Cardiff acne disability index score did not correlate with the severity of acne as assessed by global acne grading system in our study population as well as in several recent studies.[17],[24],[25],[26] Similar results were reported in a French study that assessed clinical severity using the Echelle de Cotation des L´esions d'acn´e scale.[27] A Turkish study, too, did not reveal any significant correlation of the severity of acne with acne quality of life scale and/or dermatology life quality index.[7] In addition, anxiety or depression (assessed by hospital anxiety and depression scale) was reported not to correlate with acne severity based on the global acne grading system.[28] On the other hand, a positive correlation between Cardiff acne disability index, other quality of life measurements and clinical severity of acne has been reported in other studies.[8],[19],[23]
Conclusion
Our study identified two patient groups, progressively older people having acne and people consuming alcohol and/or smoking cigarettes with a significantly greater impairment of the quality of life and thereby needing extra sensitivity on the part of physicians.
The principal reason underlying the lack of correlation between the quality of life and clinical severity in our study is most likely due to the increasing impairment of the quality of life despite lessening of clinical severity with increasing age.
While patients of acne are clinically treated as per the grading of their lesions, possible impairment in their quality of life needs to be assessed and, if required, appropriately redressed. This could be by in-depth counseling and psychotherapy including, where required, psychopharmacotherapy by a psychiatrist. Cardiff acne disability index which can give a better insight within minutes into the individual patient's psyche should be used more often and each patient should be treated individually, as even mild disease may be disproportionately distressing to some.
Acknowledgments
The authors would like to thank Professor Andrew Finlay, Department of Dermatology and Wound Healing, Cardiff University School of Medicine, Cardiff, UK.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet 2012;379:361-72.
[Google Scholar]
|
| 2. |
Zouboulis CC. Acne as a chronic systemic disease. Clin Dermatol 2014;32:389-96.
[Google Scholar]
|
| 3. |
Centers for Disease Control. Classifications of diseases and functioning and disability. In: Classifications of Diseases and Functioning and Disability. Definition of Disability Reference. Maryland: National Center for Health Statistics; 2001.
[Google Scholar]
|
| 4. |
Tasoula E, Gregoriou S, Chalikias J, Lazarou D, Danopoulou I, Katsambas A, et al. The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. Results of a population survey. An Bras Dermatol 2012;87:862-9.
[Google Scholar]
|
| 5. |
Do JE, Cho SM, In SI, Lim KY, Lee S, Lee ES. Psychosocial aspects of acne vulgaris: A community-based study with Korean adolescents. Ann Dermatol 2009;21:125-9.
[Google Scholar]
|
| 6. |
Mulder MM, Sigurdsson V, van Zuuren EJ, Klaassen EJ, Faber JA, de Wit JB, et al. Psychosocial impact of acne vulgaris. Evaluation of the relation between a change in clinical acne severity and psychosocial state. Dermatology 2001;203:124-30.
[Google Scholar]
|
| 7. |
Ilgen E, Derya A. There is no correlation between acne severity and AQOLS/DLQI scores. J Dermatol 2005;32:705-10.
[Google Scholar]
|
| 8. |
Jones-Caballero M, Chren MM, Soler B, Pedrosa E, Peñas PF. Quality of life in mild to moderate acne: Relationship to clinical severity and factors influencing change with treatment. J Eur Acad Dermatol Venereol 2007;21:219-26.
[Google Scholar]
|
| 9. |
Gupta A, Sharma YK, Dash K, Verma S. Cultural adaptation of the Cardiff Acne Disability Index to a Hindi speaking population: A pilot study. Indian J Dermatol 2015;60:419.
[Google Scholar]
|
| 10. |
Motley RJ, Finlay AY. Practical use of a disability index in the routine management of acne. Clin Exp Dermatol 1992;17:1-3.
[Google Scholar]
|
| 11. |
Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol 1997;36:416-8.
[Google Scholar]
|
| 12. |
Rubinow DR, Peck GL, Squillace KM, Gantt GG. Reduced anxiety and depression in cystic acne patients after successful treatment with oral isotretinoin. J Am Acad Dermatol 1987;17:25-32.
[Google Scholar]
|
| 13. |
Picardi A, Abeni D, Melchi CF, Puddu P, Pasquini P. Psychiatric morbidity in dermatological outpatients: An issue to be recognized. Br J Dermatol 2000;143:983-91.
[Google Scholar]
|
| 14. |
Fritzsche K, Ott J, Zschocke I, Scheib P, Burger T, Augustin M. Psychosomatic liaison service in dermatology. Need for psychotherapeutic interventions and their realization. Dermatology 2001;203:27-31.
[Google Scholar]
|
| 15. |
Magin PJ, Adams J, Heading GS, Pond CD. Patients with skin disease and their relationships with their doctors: A qualitative study of patients with acne, psoriasis and eczema. Med J Aust 2009;190:62-4.
[Google Scholar]
|
| 16. |
Koo J. The psychosocial impact of acne: Patients' perceptions. J Am Acad Dermatol 1995;32(5 Pt 3):S26-30.
[Google Scholar]
|
| 17. |
Law MP, Chuh AA, Lee A, Molinari N. Acne prevalence and beyond: Acne disability and its predictive factors among Chinese late adolescents in Hong Kong. Clin Exp Dermatol 2010;35:16-21.
[Google Scholar]
|
| 18. |
Behnam B, Taheri R, Ghorbani R, Allameh P. Psychological impairments in the patients with acne. Indian J Dermatol 2013;58:26-9.
[Google Scholar]
|
| 19. |
El-Khateeb EA, Khafagy NH, Abd Elaziz KM, Shedid AM. Acne vulgaris: Prevalence, beliefs, patients' attitudes, severity and impact on quality of life in Egypt. Public Health 2014;128:576-8.
[Google Scholar]
|
| 20. |
Stern RS. Medication and medical service utilization for acne 1995-1998. J Am Acad Dermatol 2000;43:1042-8.
[Google Scholar]
|
| 21. |
Jankovic S, Vukicevic J, Djordjevic S, Jankovic J, Marinkovic J, Basra MK. The Cardiff Acne Disability Index (CADI): Linguistic and cultural validation in Serbian. Qual Life Res 2013;22:161-6.
[Google Scholar]
|
| 22. |
Walker N, Lewis-Jones MS. Quality of life and acne in Scottish adolescent schoolchildren: Use of the Children's Dermatology Life Quality Index (CDLQI) and the Cardiff Acne Disability Index (CADI). J Eur Acad Dermatol Venereol 2006;20:45-50.
[Google Scholar]
|
| 23. |
Aghaei S, Mazharinia N, Jafari P, Abbasfard Z. The Persian version of the Cardiff Acne Disability Index. Reliability and validity study. Saudi Med J 2006;27:80-2.
[Google Scholar]
|
| 24. |
Kokandi A. Evaluation of acne quality of life and clinical severity in acne female adults. Dermatol Res Pract 2010;2010. pii: 410809.
[Google Scholar]
|
| 25. |
Basak PY, Ergin S. Effects of acne vulgaris on quality of life. Turkderm 2000;34:107-9.
[Google Scholar]
|
| 26. |
Motley RJ, Finlay AY. How much disability is caused by acne? Clin Exp Dermatol 1989;14:194-8.
[Google Scholar]
|
| 27. |
Dreno B, Alirezai M, Auffret N, Beylot C, Chivot M, Daniel F, et al. Clinical and psychological correlation in acne: Use of the ECLA and CADI scales. Ann Dermatol Venereol 2007;134 (5 Pt 1):451-5.
[Google Scholar]
|
| 28. |
Aktan S, Ozmen E, Sanli B. Anxiety, depression, and nature of acne vulgaris in adolescents. Int J Dermatol 2000;39:354-7.
[Google Scholar]
|
Fulltext Views
9,000
PDF downloads
3,743





