Translate this page into:
Recurrent cutaneous leiomyosarcoma of the inner thigh
2 Section of Pathological Anatomy, Department of Emergencies and Organ Transplantation, University of Bari, 11, Piazza Giulio Cesare, 70124, Bari, Italy
3 Section of Plastic and Reconstructive Surgery, Department of Emergencies and Organ Transplantation, University of Bari, 11, Piazza Giulio Cesare, 70124, Bari, Italy
Correspondence Address:
Michelangelo Vestita
Resident physician in Dermatology, 11, Piazza Giulio Cesare, 70124, Bari
Italy
| How to cite this article: Bonamonte D, Vestita M, Filoni A, Ingravallo G, Sportelli P. Recurrent cutaneous leiomyosarcoma of the inner thigh. Indian J Dermatol Venereol Leprol 2015;81:309-311 |
Sir,
A 55-year-old African male from Senegal presented with a large nodular lesion on the inner surface of the left thigh [Figure - 1]. The lesion had first appeared 8 years ago, had been interpreted as a keloid and excised twice once 6 years ago and again 3 years ago in Senegal without any histological examination. Clinically, the lesion measured 45 × 55 mm, was hard in consistency, moved easily over the deep subcutaneous planes, and seemed to comprise of separate, coalescing nodules. The overlying epidermis was adherent. Bilateral inguinal lymph nodes were palpable but normal. Patient reported feeling a stabbing pain in the nodule. Complete blood examination, human immunodeficiency virus antibodies, hepatitis markers, and human herpesvirus 8 polymerase chain reaction (PCR) from blood showed no abnormalities. Mycobacterial polymerase chain reaction from the skin biopsy specimen was also negative.
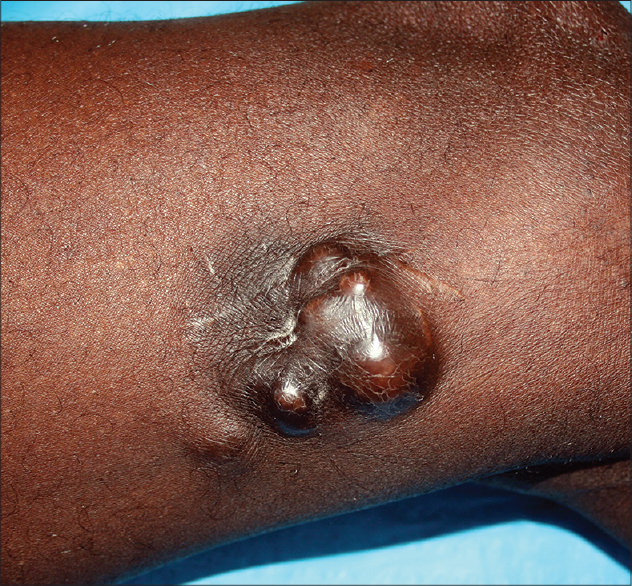 |
| Figure 1: The nodular aspect of the tumor is clearly visible |
Histology showed a dermal neoplasm partially extending into the underlying subcutaneous tissue, with a nodular growth pattern, consisting of a highly cellular tumor composed of interlacing bundles of spindle cells with eosinophilic cytoplasm and typical blunt ended nuclei [Figure - 2]a and b. The tissue involvement ratio was >90% dermal and <10% subcutaneous. Tumor cells demonstrated nuclear hyperchromasia, pleomorphism and readily identified mitoses, equivalent to 3 per 10 high-power fields. Further, they showed consistent intra-cytoplasmic immunoreactivity for desmin, vimentin, smooth muscle actin [Figure - 2]c, and actin HHF-35. More than 20% tumor cells displayed nuclear anti-Ki 67 (MIB 1) positivity. Immunoreactivities for S-100 protein, CD31 and CD34 were not detected in tumor cells. Based on the histological findings, we made a diagnosis of primary dermal leiomyosarcoma. In order to assess the local extension of the lesion, as well as possible systemic involvement, a left leg magnetic resonance imaging and a total body computed tomography scan were performed. The magnetic resonance imaging revealed a suprafascial nonhomogeneous mass measuring 47 × 58 mm. The computed tomography scan demonstrated no sign of metastasis.
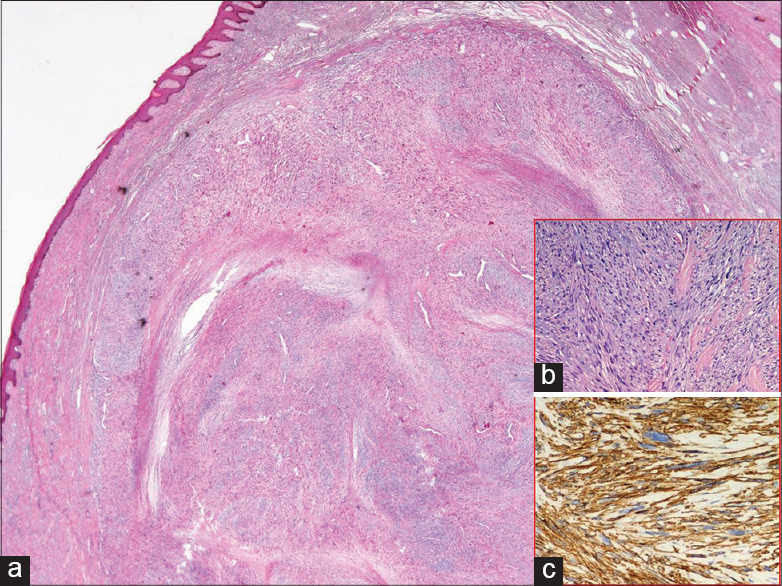 |
| Figure 2: (a) The cutaneous neoplasm displays a nodular growth pattern (H and E, ×10); (b) interlacing bundles of spindle cells (H and E, ×100); (c their immunoreactivity to smooth muscle actin (SMA) (Immunoperoxidase stain, ×200). Note the nuclear pleomorphism |
Cutaneous leiomyosarcoma is a malignant neoplasm arising from smooth muscle which affects individuals of all ages and especially between the fifth and seventh decade of life. [1] It may be subdivided into two main categories: primary and secondary. The former is rare, accounts for 2-3% of all superficial soft tissue sarcomas and includes two subtypes: dermal and subcutaneous, according to the predominantly involved tissue. [1] This implies prognostic significance as the dermal variant is a locally aggressive tumor with frequent recurrence (30-50% of cases) but it very unusually metastasizes, while the subcutaneous variant is associated with a higher percentage of metastasis and recurrence rates (50-70% of cases). [2],[3] Dermal leiomyosarcoma may involve different anatomical sites: 50-75% of cases occur on the lower limbs, predominantly on the thigh, 20-30% on the upper limbs, 10-15% on the trunk and less than 5% on the face. [2],[4] Trauma and radiation exposure have been indicated as predisposing factors.
Clinically, it can be misinterpreted as a keloid, a granulomatous lesion (such as cutaneous tuberculosis or another mycobacteriosis, cutaneous deep fungal infection, cutaneous sarcoidosis) or a neoplastic lesion both benign (epidermoid cyst, dermatofibroma, lipoma, fibroma, leiomyoma, neurofibroma) and malignant (basal cell and squamous cell carcinoma, melanoma, Kaposi sarcoma as well as other sarcomatoid tumors, notably dermatofibrosarcoma protuberans) . Histologically, it has to be differentiated from fibrosarcoma, melanoma, malignant peripheral nerve sheath tumor, synovial sarcoma, malignant fibrous histiocytoma, the spindle cell variant of squamous cell carcinoma and dermatofibrosarcoma protuberans. [5] Immunoistochemistry (smooth muscle actin, desmin, vimentin, cytokeratins, and S-100 protein) should be used. As a rule, expression of smooth muscle actin, desmin and vimentin by tumour cells and negativity for the remaining markers permits a differentiation from the above mentioned neoplasms. [3],[4] In particular, dermatofibrosarcoma protuberans was excluded in our case because neoplastic cells were CD34 negative, not arranged in a storiform pattern and the mitotic activity was very high. Guidelines for surgical intervention are not clearly defined. Recommended excision margins range from 3 to 5 cm (down to the fascia) and there are no recommendations on follow-up. [4] Local excision performed without adequate margins leads to recurrence, as had been the case with our patient.
In conclusion, primary dermal leiomyosarcoma represents a very rare entity. It should be suspected by the clinician assessing a nodular cutaneous lesion, especially in case of previous trauma or radiation, as well as by the pathologist facing a cutaneous malignant spindle cell neoplasm.
| 1. |
Jensen ML, Jensen OM, Michalski W, Nielsen OS, Keller J. Intradermal and subcutaneous leiomyosarcoma: A clinicopathological and immunohistochemical study of 41 cases. J Cutan Pathol 1996;23:458-63.
[Google Scholar]
|
| 2. |
Tsutsumida A, Yoshida T, Yamamoto Y, Itoh T, Minakawa H, Sugihara T. Management of superficial leiomyosarcomas: A retrospective study of 10 cases. Plast Reconstr Surg 2005;116:8-12.
[Google Scholar]
|
| 3. |
Bellezza G, Sidoni A, Cavaliere A, Scheibel M, Bucciarelli E. Primary cutaneous leiomyosarcoma: A clinicopathological and immunohistochemical study of 7 cases. Int J Surg Pathol 2004;12:39-44.
[Google Scholar]
|
| 4. |
Porter CJ, Januszkiewicz JS. Cutaneous leiomyosarcoma. Plast Reconstr Surg 2002;109:964-7.
[Google Scholar]
|
| 5. |
Annest NM, Grekin SJ, Stone MS, Messingham MJ. Cutaneous leiomyosarcoma: A tumour of the head and neck. Dermatol Surg 2007;33:628-33.
[Google Scholar]
|
Fulltext Views
2,978
PDF downloads
1,764





