Translate this page into:
Reinduction as a strategy to regain disease control in secondary failure to secukinumab
Corresponding author: Dr. Shekhar Neema, Department of Dermatology, Base Hospital, Lucknow, India. shekharadvait@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Neema S, Pathania V, Nyalam P, GB Prashanth, Kamboj P. Reinduction as a strategy to regain disease control in secondary failure to secukinumab. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1961_2024
Dear Editor,
Psoriasis is a chronic, immune-mediated inflammatory dermatosis with a prevalence ranging from 0.44 to 2.8% in India. Secukinumab is a fully human IgG1κ, anti-IL-17A monoclonal antibody, approved by the FDA in 2015 for treating moderate to severe plaque psoriasis. Secukinumab achieves psoriasis area and severity index (PASI) 75/90/100 responses in approximately 90%, 79%, and 63% of patients at 16 weeks, and 79%, 72%, and 55% at 136 weeks, respectively.1 Treatment failures with secukinumab are uncommon and can be classified as primary or secondary. Primary failure is defined as an insufficient initial response (failure to achieve PASI75 within 12 -16 weeks), while secondary failure is the loss of disease control after achieving it.2 Secondary failure poses a clinical challenge, particularly in patients with prior inadequate responses to conventional therapies and in settings where newer IL-23 or IL-12/23 inhibitors are not available. Here, we describe two cases of secondary failure successfully treated with reinduction.
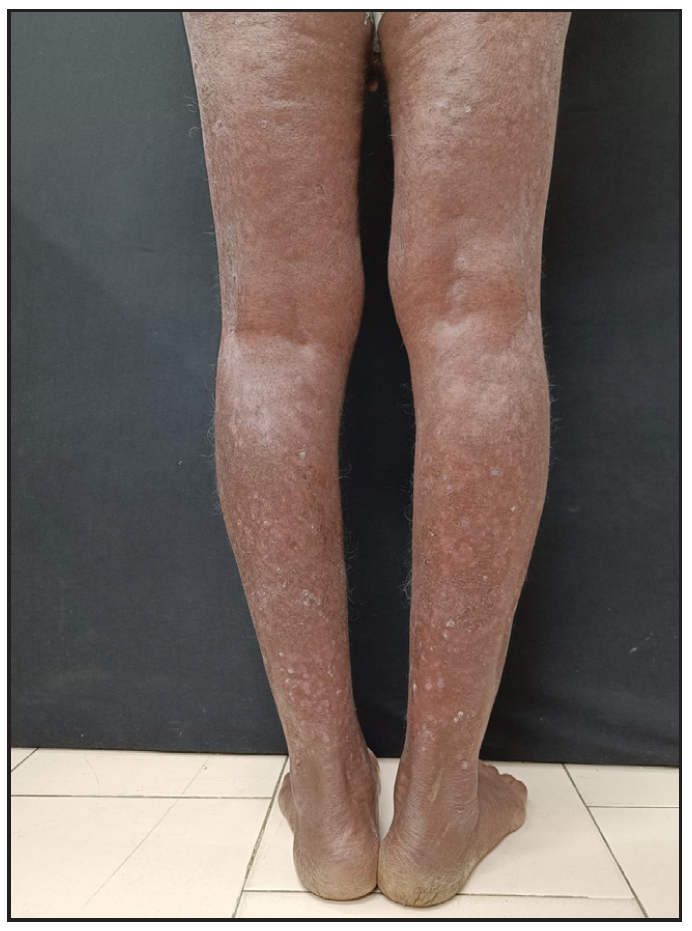
A 71-year-old man with chronic plaque psoriasis for thirty years presented with lesions involving ∼70% of the body surface area (BSA) and PASI48. He had been treated with methotrexate, cyclosporine, apremilast, acitretin, etanercept, adalimumab, and phototherapy over those 30 years. He was treated with the standard dosage (300 mg 0,1,23,4 weeks and then 4 weekly) of injection secukinumab. The patient showed significant improvement, achieving PASI 90 at 12 weeks, which was maintained until week 32, after which disease control began to decline. By week 40, plaques reappeared, involving 40% BSA with a PASI of 26, despite regular maintenance dosing. [Figure 1a]. The patient was diagnosed with secondary failure, and re-induction with secukinumab was given (300 mg weekly for 4 weeks). At 12 weeks, the PASI reduced to 4 and remained the same at the 12-month follow-up [Figure 1b].

- Secondary failure in a patient on secukinumab presenting as 40% body surface area involvement with Psoriasis area and severity index 26.
- Reduction to psoriasis area and severity index 4, 12 weeks after reinduction with secukinumab.
A 34-year-old man with chronic plaque psoriasis with psoriatic arthritis for 9 years presented with a flare of 1 month. He was treated with methotrexate, cyclosporine, phototherapy, and adalimumab. He was given secukinumab due to a secondary failure to adalimumab. He responded well to treatment and maintained remission for 40 weeks. Despite regular maintenance dosages, he developed secondary failure to secukinumab, resulting in 70% BSA and PASI35 [Figure 2a]. Reinduction was given with secukinumab. The individual responded well, achieving a PASI of 4 [Figure 2b] at 8 weeks and complete clearance of lesions over the next 8 weeks, which was maintained at the 1-year follow-up.
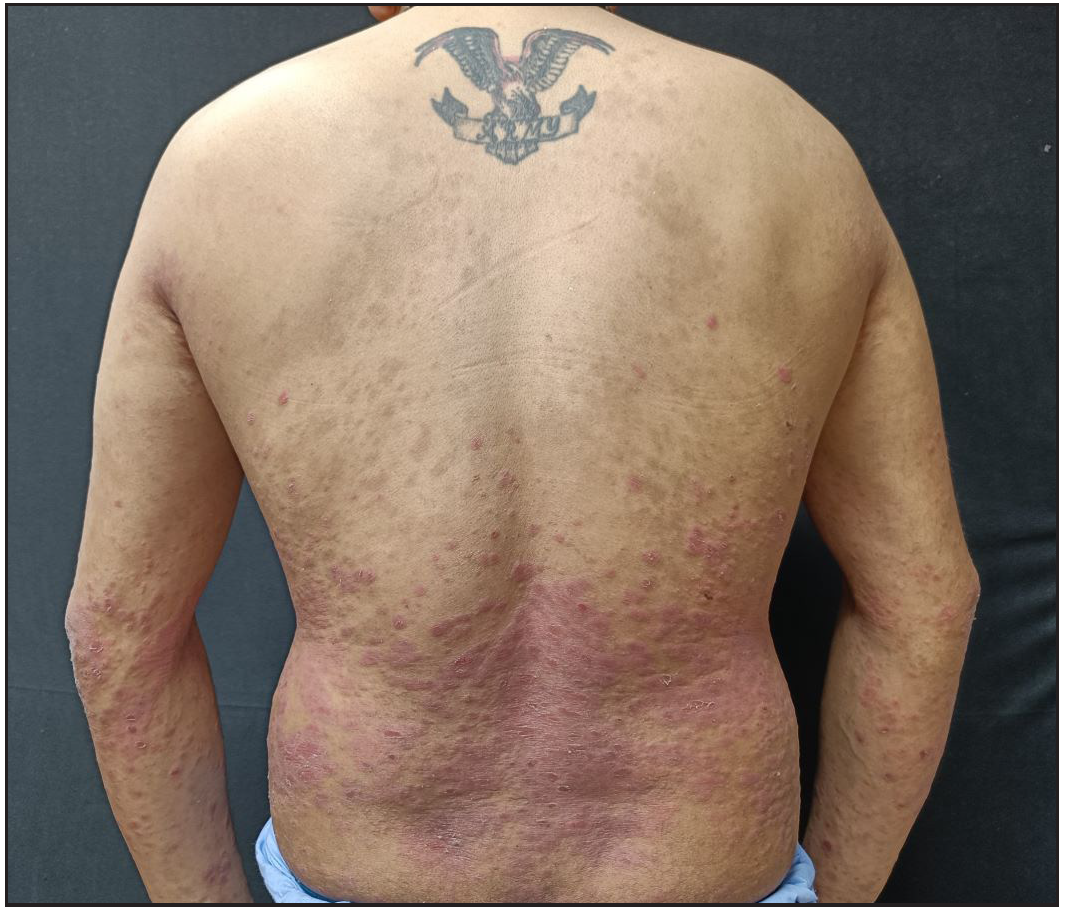
- Secondary failure in a patient on secukinumab presenting with 70% body surface area involvement.
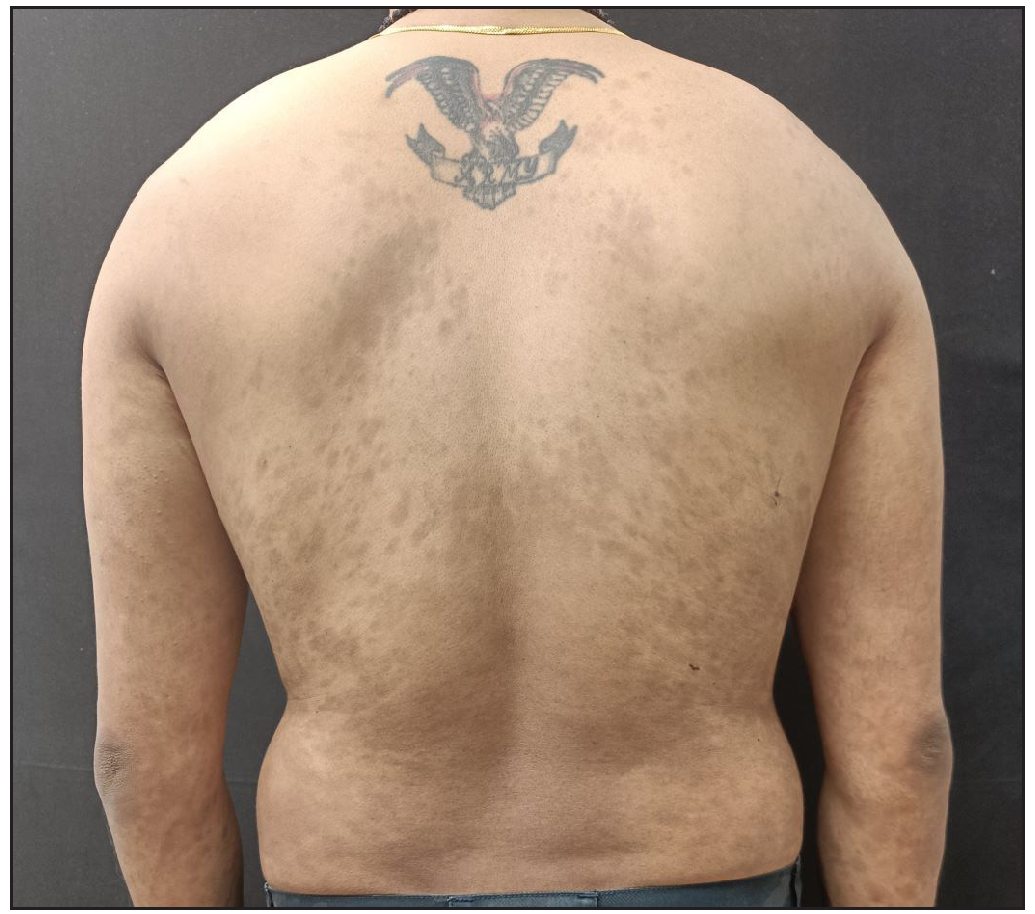
- Clearance of skin lesions 8 weeks after reinduction.
Secondary failure is an important clinical problem in psoriasis management and is seen with conventional as well as biologic therapy. Drug survival (the time for which the patient continues treatment) varies depending on the medications. Biologics therapy has an overall longer survival time than conventional treatment. Adverse events are a major reason for discontinuation of conventional treatments and may also contribute to secondary failure of biologics.3 In a study by Shin et al., 80% of patients switched biologics due to secondary failure, and 5% due to primary failure and adverse events. The class of biologics was switched in secondary failure, and similar PASI75/90/100 was seen after administration of the second biologic (secukinumab, ustekinumab, or adalimumab).3
Ting et al. found that primary failure was the most common reason for discontinuing etanercept (42%) and adalimumab (22%), while secondary failure caused discontinuation of secukinumab (18%) and infliximab (14.3%).4 The drug survival of IL-17 inhibitors is higher in biological naïve than experienced patients. The exact cause of the secondary failure is unknown. Potential causes include the development of anti-drug antibodies (increasing drug clearance), shifts in immunological pathways (e.g., increased Tumour necrosis factor (TNF)-α production in patients on IL-17 inhibitors), lifestyle changes such as weight gain, or the influence of concomitant medications.5 The treatment strategies for secondary failure are dose escalation, switching biologics, and switching to or adding conventional therapy. The dose escalation strategy has been used with adalimumab (increased to 40 mg weekly) in primary and secondary failure, demonstrating adequate response.6 Class switching is an effective therapeutic option, such as switching to IL-12/23 or IL-23 inhibitors when IL-17 inhibitors fail. Intra-class switching can also be done. Ixekizumab may be effective in secukinumab failure, due to its higher affinity to IL-17A and its ability to target different epitopes.7 Switching the biologic class is a viable option; however, some biologics are unavailable in our country, and switching to conventional agents is limited by previous adverse effects, intolerance, or poor efficacy. Reinduction or dose escalation increases the trough levels of biologic and may resume disease control. However, dose escalation may be more expensive than introducing a new class of biologics. Until newer classes of biologics become available in our country, dose escalation or reinduction is a reasonable strategy to regain disease control, especially in patients showing contraindications to conventional therapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- In which patients the best efficacy of secukinumab? Update of a real-life analysis after 136 weeks of treatment with secukinumab in moderate-to-severe plaque psoriasis. Expert Opin Biol Ther. 2020;20:173-182.
- [CrossRef] [PubMed] [Google Scholar]
- Biologic therapy for moderate to severe psoriasis Real-world follow-up of patients who initiated biologic therapy at least 10 years ago. Dermatol Ther (Heidelb). 2022;12:761-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Review of the reasons for and effectiveness of switching biologics for psoriasis treatment in korea. Indian J Dermatol Venereol Leprol. 2023;89:928.
- [CrossRef] [PubMed] [Google Scholar]
- Drug survival of biologics in psoriasis: An australian multicentre retrospective study. Australas J Dermatol. 2024;65:350-7.
- [CrossRef] [PubMed] [Google Scholar]
- Drug survival outcomes associated with the real-world use of ixekizumab, secukinumab, guselkumab, and adalimumab for the treatment of plaque psoriasis in China: A 52-week single-center retrospective study. Clin Cosmet Investig Dermatol. 2022;15:2245-52.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effectiveness of adalimumab dose escalation, combination therapy of adalimumab with methotrexate, or both in patients with psoriasis in daily practice. J Dermatolog Treat. 2013;24:361-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment with ixekizumab following secukinumab failure in patients with psoriatic arthritis: Real-life experience from a resistant population. Biologics. 2021;15:463-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





