Translate this page into:
Relapse in psoriasis with two different tapering regimens of methotrexate: A randomized open-label controlled study
2 Department of Dermatology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, India
Correspondence Address:
Satyendra Kumar Singh
Department of Dermatology and Venereology, Institute of Medical Sciences, Banaras Hindu University, Varanasi - 221 005
India
| How to cite this article: Singh SK, Rai T. Relapse in psoriasis with two different tapering regimens of methotrexate: A randomized open-label controlled study. Indian J Dermatol Venereol Leprol 2015;81:144-147 |
Abstract
Background : Systemic therapy with methotrexate is a very useful modality in psoriasis, but relapses can occur soon after stopping it. Aim : To compare the relapse rates in psoriasis with two different tapering regimens of methotrexate after control is achieved. Methods : This was a randomized open-label controlled study, and patients of chronic plaque psoriasis with psoriasis area and severity index (PASI) >10 were included. Methotrexate 0.3 mg/kg weekly was given and the PASI calculated every 2 weeks. After achieving a 75% reduction in the PASI (PASI-75), patients were assigned randomly in to one of three groups. In the half-dose group, the dose of methotrexate was reduced to half and given weekly; in the 2-weekly group, the same dose was given at 2-week intervals; in the control group, methotrexate was stopped. Patients were followed up for 12 weeks. Results : Out of 141 registered patients, 81 were included: 27 in the half-dose group, 28 in the 2-weekly group, and 26 in the control group. After further exclusions due to adverse effects and loss to follow-up, the results were analysed for 16, 17 and 19 patients respectively in the 3 groups. There was statistically a highly significant difference in relapse rates between the half-dose and control groups (P < 0.001), and a significant difference between the 2-weekly and control groups (P = 0.001). Relapse rates in the half-dose and 2-weekly groups did not show a significant difference (P = 0.680). Limitation: Many (35.8%) patients were excluded and only 52 (64.2%) completed the study. Conclusion: There appears to be no significant difference in the frequency of relapse in psoriasis whether methotrexate is tapered by halving the weekly dose or by doubling the interval between two doses, and both methods led to fewer relapses than abrupt cessation of the drug.INTRODUCTION
Psoriasis is a chronic, inflammatory, hyperproliferative skin disease. Plaque psoriasis is the most common form, accounting for approximately 90% of cases. Exacerbating factors include infection, endocrine factors, hypocalcaemia, medications, psychological stress and skin trauma. There being no cure, the aim of treatment is to minimize the extent and severity of the disease so that it no longer substantially impairs the patient′s quality of life. Patients who discontinue treatments may experience a return of disease (relapse) or worsening of disease (rebound). [1]
Methotrexate has been used successfully in the treatment of severe chronic plaque psoriasis for many years, and is also particularly beneficial in psoriatic arthritis as well as in acute forms of the disease, such as erythrodermic or pustular psoriasis. It has been shown to achieve PASI-75 in 60% of patients, [2] but discontinuation often leads to a relapse. Studies on how to taper off the dose of methotrexate are lacking. Whether the dose should be decreased or the interval between two doses increased is not clear. The present study attempts to look at the relapse rates in psoriasis with two different tapering regimens of methotrexate after achieving PASI-75. We were unable to find any previous published studies that addressed this question.
METHODS
The study was designed as a randomized open-label controlled trial and approved by the Institutional Ethics Committee. It was conducted at the Department of Dermatology and Venereology, Institute of Medical Sciences, Banaras Hindu University, a tertiary health care centre in Varanasi, from September 2010 to April 2012. No blinding was attempted and patients bought their own medication. The sample size was determined by presuming a relapse rate of 50% after achieving PASI-75 in the control group wherein treatment was stopped abruptly, and 10% in the other two groups. The level of significance was taken as 5% and the power of the test was 90%. Taking a one-to-one ratio in each group, the minimum sample size was found to be 25 for each group.
Inclusion criteria were: patients with severe psoriasis of age 18 to 65 years, PASI >10, no topical treatment 7 days prior and no systemic treatment in the 30 days prior to inclusion, and a cumulative dose of methotrexate <1 g. Exclusion criteria were: pregnancy, lactation, alcohol dependence, a history of jaundice, hemoglobin <8 g/dl, total leukocyte count <4000 cells/mm 3 , platelet count <1 lakh/mm 3 , lymphocyte count <1500 cells/mm 3 , liver transaminases >100 U/ml, renal disease, tuberculosis, immunosupression, and patients who did not seem to understand instructions.
Methotrexate was prescribed in a dose of 0.3 mg/kg weekly to all patients till PASI-75 was achieved. Patients were asked to follow up every 2 weeks for 12 weeks and the PASI was determined at each visit by the same investigator. Complete blood counts and liver function tests were repeated every 2 weeks, and renal function tests every 2 months. Patients were also asked about other side effects of methotrexate like nausea, vomiting, black stools, oral ulceration, dry cough, and loss of appetite. In case of severe adverse effects, methotrexate was discontinued.
All subjects received the same treatment until they achieved PASI-75. Patients who did not achieve PASI-50 in 3 months were put on other systemic treatments and excluded, while those with scores between PASI-50 and PASI-75 at 3 months were treated till they achieved PASI-75. Those who achieved PASI-75 were allocated to one of the following groups according to a random number sequence that was generated by a colleague.
Half-dose group: The dose of methotrexate was reduced to half the original dose (1.5 mg/kg), weekly.
Two-weekly group: Methotrexate was advised at the same dose (0.3 mg/kg) but at every 2 weeks.
Control group: Methotrexate was discontinued.
All patients were followed up every 2 weeks for the next 3 months and evaluated for relapses, defined as a loss of 50% PASI improvement from the baseline (i.e, a return to PASI 37.5) in patients who had achieved a clinically meaningful response (PASI-75). Laboratory monitoring was also continued in the patients in the treatment groups.
Statistical analysis
The various parameters observed were compared using Chi-square test for noncontinuous variables and analysis of variance (ANOVA) for continuous variables.
RESULTS
A total of 141 patients of severe plaque psoriasis were registered, of whom 60 had to be excluded because of adverse effects or loss to follow-up [Figure - 1]. Eighty-one patients were included: 27 in the half-dose group, 28 in the 2-weekly group, and 26 controls. Of these, only 16, 17 and 19 patients in the three groups respectively were included in the analysis, as the rest were again lost to follow-up or could not continue in the study due to adverse effects.
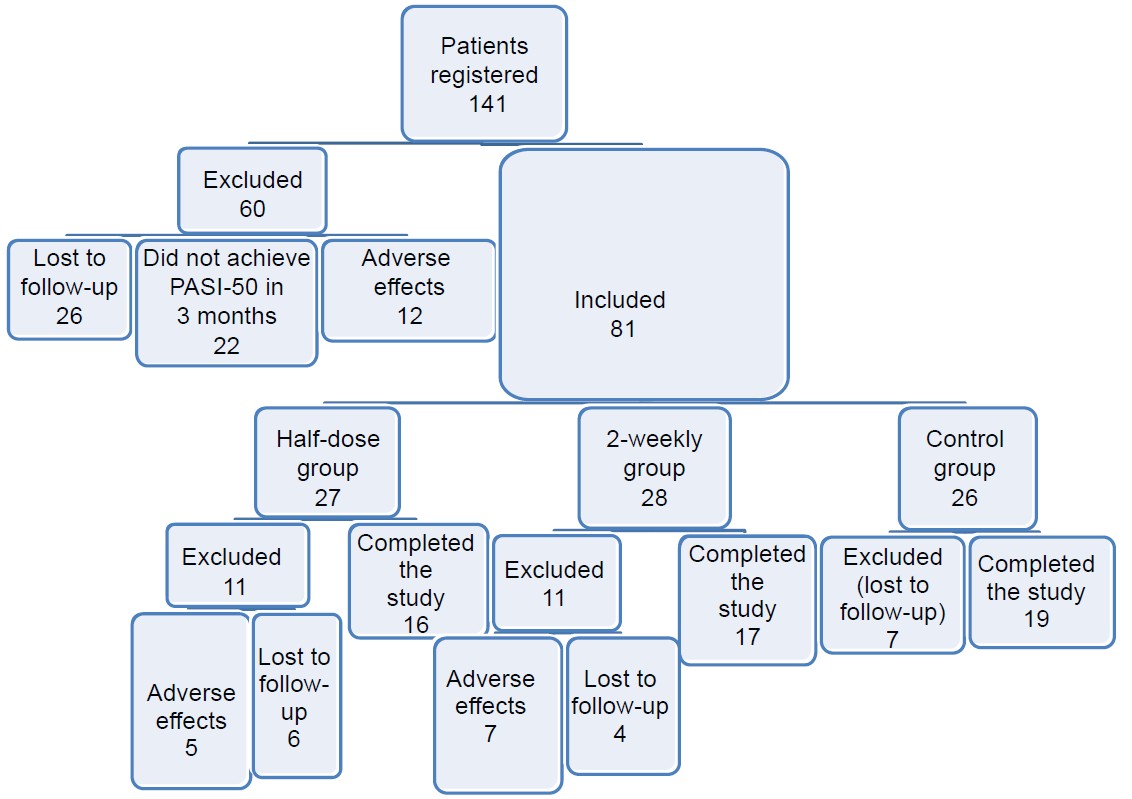 |
| Figure 1: Flow of patients through the study |
The groups were matched for age (mean ± SD, 39.26 ± 15.98, 33.36 ± 12.82 and 38.64 ± 15.19 years respectively, P = 0.77) and sex distribution (P = 0.57). The mean duration of disease was 10.40 + 3.86 in the half-dose group, 9.76 + 3.99 in the 2-weekly group, and 7.68 + 3.00 in the control group. All three groups were also matched for severity of disease at the beginning of treatment (mean PASI ± SD 19.90 ± 10.64, 20.44 ± 12.28 and 19.95 ± 9.80 respectively, P = 0.98 and F-value = 0.020).
All patients included reported on time for follow-up assessments. PASI-75 was attained at 4-30 weeks (mean ± SD, 9.48 ± 4.17 weeks). The median period to achieve PASI-75 was 8 weeks in all three groups (χ2 = 0.33, df = 2, P = 0.89). Two (12.5%) patients in the half-dose group, 3 (17.6%) in the 2-weekly group and 14 (73.7%) patients in the control group experienced relapses. There was no statistically significant difference in relapse rates between the half-dose and 2-weekly groups (P = 0.680), but the difference between the half-dose and control groups was highly significant (P < 0.001). Relapse rates were also significantly different between the 2-weekly and control groups (P = 0.001).
Adverse effects of methotrexate led to 12 patients in the two tapered dose groups being excluded. Nausea and vomiting were the most common side-effects (8 patients), mainly on the day of methotrexate administration, though in 2 patients they continued for 2-3 days. Oral folic acid (5 mg/day) improved the symptoms in 7 of these patients, but one had severe vomiting not controlled even with ondansetron injections, and methotrexate had to be stopped. Methotrexate was also discontinued in 4 patients who developed thrombocytopenia within 2 weeks; the platelet count subsequently normalized in these patients. Elevation of liver transaminases was seen in 12 patients, and methotrexate was stopped in 7 of these, since the increase was more than twice the baseline value. Two patients had mild elevations of serum bilirubin. There were no apparent common factors amongst the cases where methotrexate had to be discontinued due to side effects.
Another 12 patients were excluded due to side effects before being assigned to any of the study groups: Anemia with hemoglobin <8 g/dl (1 patient), serum bilirubin >30% above baseline (2), severe headache and weakness (2), elevated liver transaminases (5), and thrombocytopenia (2).
DISCUSSION
Relapses are common in psoriasis and patients may require maintenance therapy for long durations. The pattern of relapse varies, with some patients having early and frequent relapses while others may have long-term remissions with infrequent relapses. Data on the relapse patterns of psoriasis in India are scarce. In one Indian study, the duration of remissions varied widely from 2 weeks to 9 years, and 4% of patients never had complete remission. [3] Studies on cyclosporine in psoriasis found that 50-60% of patients relapsed 6 months after treatment withdrawal, [4] and that the time to relapse depended on the severity of disease, the dose required to achieve clearance, and the extent of clearing achieved before termination of the drug. [5],[6]
At the cellular level, post-therapeutic relapse of psoriasis has been found to be associated with a rapid influx of T-cells and inflammatory myeloid dendritic cells into the skin after discontinuing efalizumab (anti-CD11a). [7] Gene set enrichment analysis of the transcriptome showed that while efalizumab induced an improvement in many psoriasis genes, during relapse, the majority of these genes reverted to their lesional state, leading to the development of typical lesions.
An established parameter to measure the severity of psoriasis is the PASI, introduced in 1978 as an outcome measure in a retinoid trial. [8] It is part of most currently used classifications of disease severity in psoriasis and represents a necessary first step in selecting a treatment strategy. In this study, we compared the relapse after tapering methotrexate in two different ways once PASI-75 was achieved. A PASI-75 response, as documented in the European S3 guidelines on the systemic treatment of psoriasis vulgaris, can be achieved in the majority of patients with moderate-to-severe disease using current treatments. Although complete clearance may be regarded as the ultimate treatment goal for psoriasis, a PASI-75 response has been proposed as a more realistic goal. In other recent clinical trials too, especially those investigating biological therapies, the most commonly used primary efficacy measure has been the PASI-75 response.
There are no clear guidelines on the duration of treatment with methotrexate in psoriasis to the best of our knowledge: should it be life-long or till the patient develops hepatotoxicity? In practice, methotrexate is sometimes continued for years, especially when the clinical response is satisfactory and there are no major adverse effects. However, a high cumulative dose of methotrexate being associated with an increased frequency of liver damage, it is advisable to reduce the dose once a clinically meaningful response is obtained. On the other hand, it is also apparent that patients of chronic psoriasis require maintenance therapy for long durations. In one report, all five patients who discontinued methotrexate after post-clearance maintenance therapy had relapsed 4-10 weeks later, while another 5 who continued on the maintenance dose (5 mg/week) did not. [9] In another study on 20 patients with chronic plaque psoriasis, methotrexate in the dose of 15 mg/week was given till remission (4-12 weeks), followed by a maintenance dose (5 mg/week) for 8 weeks. All patients were found to have relapsed within 6-12 weeks of stopping methotrexate. [10] Our patients were followed up for 12 weeks, and 19 (36.5%) out of 52 patients relapsed during this period. In our study, relapse was not universally noted by 3 months even when methotrexate was stopped abruptly. Overall, it appears that 5-7.5 mg/week maintenance doses of methotrexate lower the chances of early relapses.
A limitation in our study is that 29 (35.8%) patients were excluded and only 52 (64.2%) completed it. This is however similar to one of the above studies, where 5 out of 15 patients did not follow up or had to discontinue methotrexate because of side-effects. [9]
In conclusion, we found that abruptly stopping methotrexate after achievement of PASI-75 led to a higher relapse rate as compared to a lowering of the dose. It is therefore suggested that methotrexate may be tapered either by halving the weekly dose or by reducing the frequency to once in 2 weeks.
ACKNOWLEDGMENT
We thank Prof. T.B.Singh, Department of Biostatics, IMS, BHU for his help in generating the random number sequence.
| 1. |
Carey W, Glazer S, Gottlieb AB, Lebwohl M, Leonardi C, Menter A, et al. Relapse, rebound, and psoriasis adverse events: An advisory group report. J Am Acad Dermatol 2006;54 4 Suppl 1:S171-81.
[Google Scholar]
|
| 2. |
Heydendael VM, Spuls PI, Opmeer BC, de Borgie CA, Reitsma JB, Goldschmidt WF, et al. Methotrexate versus cyclosporine in moderate to severe chronic plaque psoriasis. N Engl J Med 2003; 349:658-65.
[Google Scholar]
|
| 3. |
Kaur I, Handa S, Kumar B. Natural history of psoriasis: A study from the Indian subcontinent. J Dermatol 1997;24:230-4.
[Google Scholar]
|
| 4. |
Pathirana D, Ormerod AD, Saiag P, Smith C, Spuls PI, Nast A, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol 2009;23:1-70.
[Google Scholar]
|
| 5. |
Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol 2009;61:451-85.
[Google Scholar]
|
| 6. |
Ellis CN, Fradin MS, Hamilton TA, Voorhees JJ. Duration of remission during maintenance cyclosporine therapy for psoriasis: Relationship to maintenance dose and degree of improvement during initial therapy. Arch Dermatol 1995;131:791-5.
[Google Scholar]
|
| 7. |
Johnson-Huang LM, Pensabene CA, Shah KR, Pierson KC, Kikuchi T, Lentini T, et al. Post-Therapeutic Relapse of Psoriasis after CD11a Blockade Is Associated with T Cells and Inflammatory Myeloid DCs. PLOS One 2012;7:e30308.
[Google Scholar]
|
| 8. |
Fredriksson T, Pettersson U. Severe psoriasis-oral therapy with a new retinoid. Dermatologica 1978; 157:238-44.
[Google Scholar]
|
| 9. |
Dhir R, Tutakne MA, Chari KVR. Relapse in psoriasis after methotrexate. Indian J Dermatol Venereol Leprol 1992;58:77-9.
[Google Scholar]
|
| 10. |
Karibasappa NA, George A. Relapse in psoriasis after methotrexate. Indian J Dermatol Venereol Leprol 1997;63:307-9.
[Google Scholar]
|
Fulltext Views
8,656
PDF downloads
2,012





