Translate this page into:
Resistance to anti-leprosy drugs: A cross-sectional study from a tertiary care hospital in Puducherry
Corresponding author: Dr. Bodicharla Manjula, Sri Manakula Vinayagar Medical College and Hospital, Kalitheerthalkuppam, Puducherry, India.manmanju619@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Manjula B, Gopinath H, Karthikeyan K. Resistance to anti-leprosy drugs: A cross-sectional study from a tertiary care hospital in Puducherry. Indian J Dermatol Venereol Leprol 2023;89:456-8.
Dear Editor,
This was a cross-sectional study conducted in the department of dermatology, venereology and leprosy and central research laboratory in Sri Manakula Vinayagar Medical College and Hospital, Puducherry. The data was collected over a period of one and a half years after approval from the institutional ethics committee. All new leprosy patients diagnosed on the basis of clinical features and histopathological examination were included in this study. Patients under treatment and those with indeterminate leprosy were excluded from the study.
A part of the skin biopsy sample was subjected to DNA extraction with concentrations varying from 0.1–1 microgram, followed by amplification by polymerase chain reaction (PCR) using primers [Table 1] of the drug-resistant determining regions in the rpoB, folP1 and gyrAgenes. The samples in which genes were amplified were subjected to DNA sequencing to detect mutations, following standard protocol for DNA extraction and PCR amplification.1
| Genes | Primer | Sequence (5'-3') |
|---|---|---|
| folP | folP-F | CTTGATCCTGACGATGCTGT |
| folP | folP-R | CCACCAGACACATCGTTGAC |
| rpoB | rpoB-F | GTCGAGGCGATCACGCCGCA |
| rpoB | rpoB-R | CGACAATGAACCGATCAGAC |
| gyrA | gyrA-F | ATGGTCTCAAACCGGTACATC |
| gyrA | gyrA-R | TACCCGGCGAACCGAAATTG |
The rpoB, folP1 and gyrA gene PCR products of Mycobacterium leprae were sequenced at EurofinsGenomics (Bengaluru, India) using ABIPRISM®BigDye Terminator and ABI 3730XL sequencer (Applied Biosystem, USA), using both forward and reverse primers mentioned previously. After sequencing, the sequence chromatogram files were examined for quality and the low-quality ends of sequences were trimmed by using bio-edit version 7.0.9 (Isis Pharmaceuticals). Species identification of the bacteria was achieved by comparing the nucleotide sequence of genes against known sequences available in the Genbank microbial genomes database using the basic local alignment search tool.2 Mutation analysis of rpoB, folP1 and gyrAgenes was done by multiple nucleotide alignment of each gene sequence. Based on the analysis, mutation at the nucleotide level and corresponding amino acid changes at the protein level were documented. The rpoB, folP1 and gyrAgenes sequences of the present study bacterial isolates were deposited in the NCBIGenBank database and accession numbers were obtained MW239611–23 and MW245059–61, respectively.3
DNA was extracted from 31 (20 male and 11 female) patients. The age of the patients ranged from 18 to 74 years (mean age 46.5 years). Primary amplification is a technique for cloning specific or targeted parts of a DNA sequence. In our study, it yielded folP1 255, rpoB 279, gyrA 225 base pairs. Of the 31 cases, 21 did not show any mutation. In 19 amplicons, rpoB was amplified in 10, folP1 in six and gyrA in three cases and when we attempted sequencing, sequencing errors occurred in three amplicons [Figures 1a to c]. Among 16 amplicons in our study, rifampicin resistance was detected in one. It was detected in a 67-year female patient who was untreated. There was no history of anti-tuberculosis therapy, chest X-ray was normal. Her bacteriological index was one and she had borderline tuberculoid Hansen's disease. Of the eight amplified samples of rpoB, one showed a mutation in 412 position coding (Ile→Asp) isoleucine to asparagine [Figure 1a]. This mutation is unique and not reported in India.
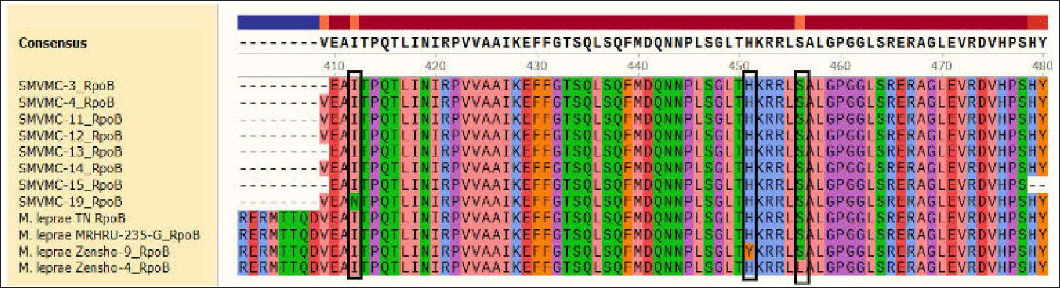
- It was a noval mutation named as SMVMC-19 at 412 position Ile 412 Asn(I412N) ATC-AAC (T1235A).Out of 8 samples of rpoB one showed mutation
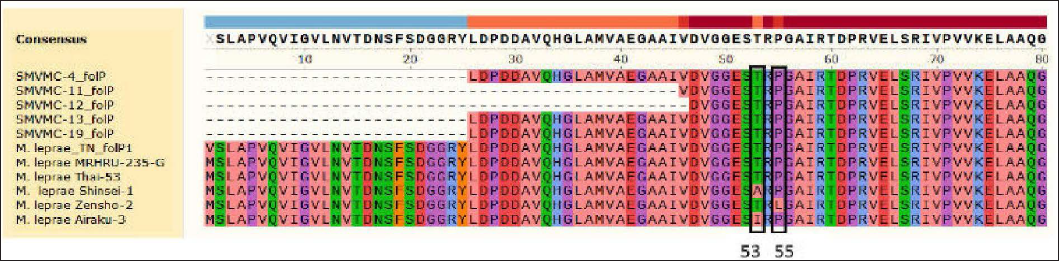
- Of five samples of folPno mutation was detected
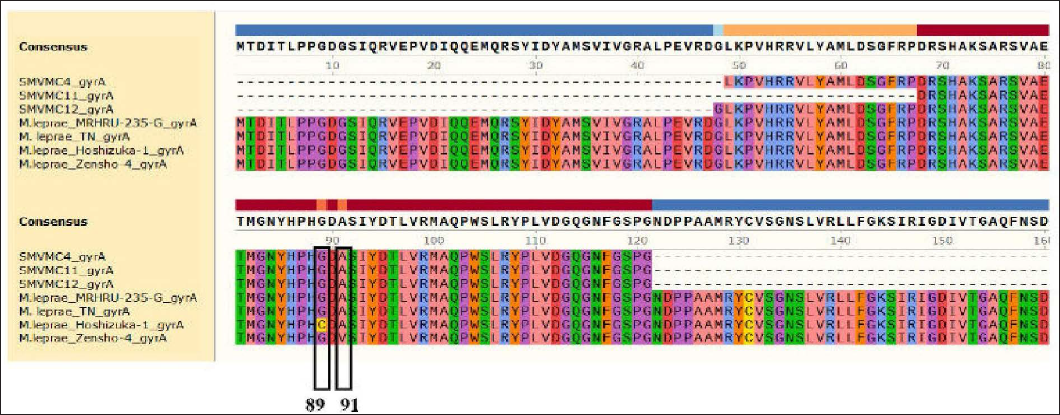
- Of the three samples of gyrAno mutation was detected
According to other previous studies, drug resistance in Mycobacterium leprae may be primarily attributed to mutations in genes encoding drug targets. For treatment and containment of drug resistance it is mandatory to have data on drug resistance.4
We are reporting rifampicin resistance in a treatment-naïve leprosy patient. The significance of our study is to establish the relevance of a drug resistance monitoring policy and careful post-treatment follow up of cured patients in order to detect relapse earlier. Without doubt, rifampicin in multidrug therapy is the standard regime for leprosy; extended therapy can be useful in secondary resistance for their inclusion in new drug regimen.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
IADVL Postgraduate thesis grant, 2018.
Conflict of interest
There are no conflicts of interest.
References
- The biosynthesis of folic acid. IX. Purification and properties of the enzymes required for the formation of dihydropteroicacid. J Biol Chem. 1969;244:1582.:92.
- [PubMed] [Google Scholar]
- 2020. GenBank microbial genomes database using the Basic Local Alignment Search Tool [Online]. [cited on Nov 2020] Available from: URL: https://blast.ncbi.nlm.nih.gov/Blast.cgi
- 2020 [Online] [cited on Nov 2020]. Available from: URL https://www.ncbi.nlm.nih.gov/Genbank/update.html
- Resistance to anti leprosy drugs in multi-bacillary leprosy: A cross-sectional study from a tertiary care centre in eastern Uttar Pradesh, India. Indian J Dermatol Venereol Leprol. 2018;84:275-9.
- [CrossRef] [PubMed] [Google Scholar]





