Translate this page into:
Retinoids as chemopreventive agents
Correspondence Address:
Sandeep Kaur
Skin OPD, OPD Block, First Floor, GGS Medical College and Hospital, Sadiq Road, Faridkot - 151 203, Punjab
India
| How to cite this article: Kaur S. Retinoids as chemopreventive agents. Indian J Dermatol Venereol Leprol 2016;82:59-64 |
Introduction
Carcinogenesis is a multistep process that transforms normal cells into malignant ones. The various steps involved are: (1) initiation, where DNA damage occurs; (2) promotion, where additional genetic mutations accumulate; and (3) progression to locally invasive or distant metastatic disease. It is believed that carcinogens produce “fields” of mutated cells long before invasive malignant disease develops; this is the fundamental basis for the concept of using medication to 'prevent' cancer. The phrase 'cancer chemoprevention' was used in the published literature for the first time by Sporn et al. in 1976.[1] Extensive epidemiological and clinical data support the role of retinoids as chemopreventive agents. Features which favour the use of retinoids for this purpose include the availability of adequate pharmacological data, experience and dose-response data, reasonably good safety profile, and a convenient dosing schedule and route of administration.
Mechanism of Retinoid Action
The retinoids are natural and synthetic derivatives of vitamin A. Upon entry into cells, retinoids bind to cytosolic retinoid-binding proteins (retinoic acid-binding proteins and retinol-binding proteins). This complex then translocates to the nucleus where retinoids bind to nuclear retinoid receptors through their DNA-binding domains, thereby resulting in altered gene expression. Two classes of nuclear retinoid receptors are known: Retinoic acid receptors (RAR - α, β, γ) and retinoid X receptors (RXR - α, β, γ). Retinoic acid receptors (RAR) form heterodimers with retinoid X receptors (RXR); retinoid X receptors can additionally form heterodimers with vitamin D or thyroid hormone receptors, or form homodimers with other retinoid X receptors. [Figure - 1] provides a diagrammatic representation of the mechanism of action of retinoids.
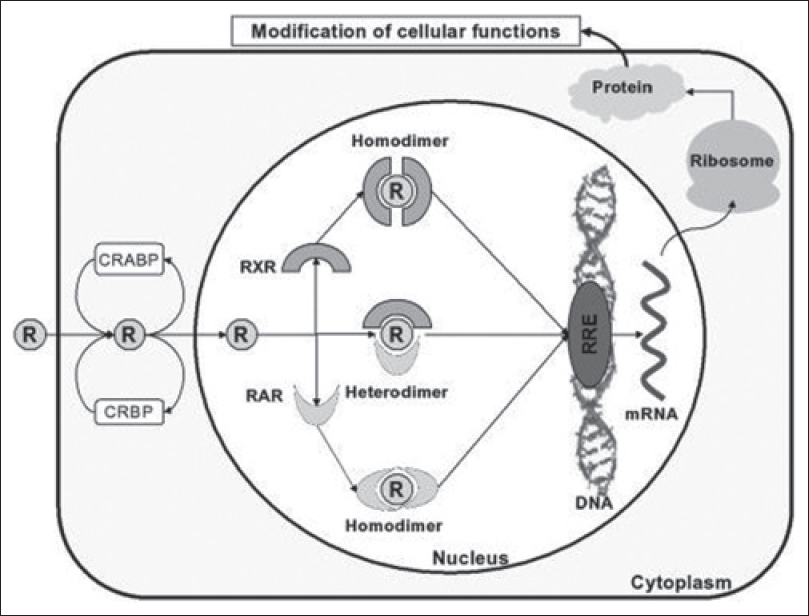 |
| Figure 1: Mechanism of action of retinoids |
Clinical Activity of Retinoids as Chemopreventive Agents
The role of retinoids in cancer prevention was first highlighted in 1925 when vitamin A was found to be required for epithelial cell homeostasis.[2] Rats rendered vitamin A-deficient developed squamous metaplasia at several epithelial sites. An inverse relationship exists between vitamin A levels and the incidence of malignancies, which provides additional evidence in favor of this role of retinoids.
Clinical data reveals that retinoids are active in reducing some second primary cancers. For example, 13-cis-retinoic acid reduces second aerodigestive tract tumors in patients with resected head and neck cancers.[3] Retinoids are also used to treat overt malignancies, including acute promyelocytic leukemia, juvenile chronic myelogenous leukemia and mycosis fungoides.
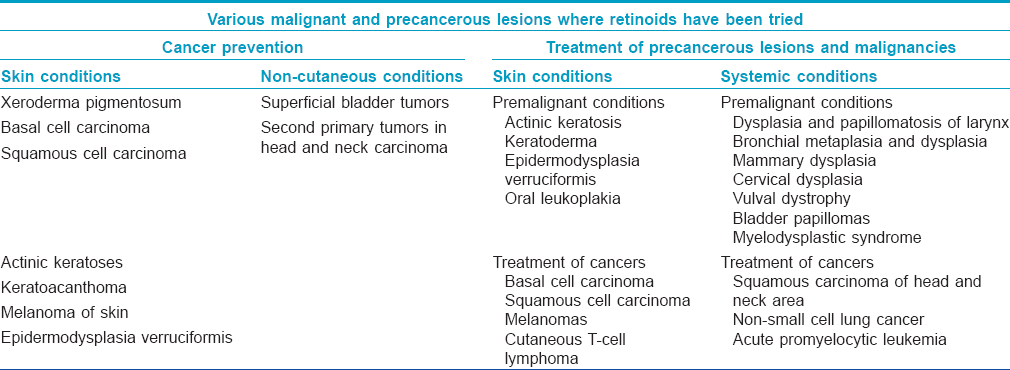
Various precancerous and malignant conditions where retinoids have been tried either for prevention or treatment are listed in [Table - 1].
Retinoid Chemoprevention Mechanisms
Experimental data suggests that retinoids act during the promotion and progression stages of carcinogenesis. Various mechanisms which contribute to their chemoprotective properties are:
- Retinoid-induced proteolysis: Retinoids cause cell cycle arrest in G1 phase via retinoid-dependent proteolysis of G1 cyclins D1 and E thus preventing phosphorylation of the retinoblastoma protein or other substrates.[4] This G1 cell cycle arrest allows repair of genomic damage caused by carcinogens
- In acute promyelocytic leukemia, retinoic acid induces degradation of the oncogenic protein, promyelocytic leukemia-retinoic acid receptor-α. This protein results from the balanced (15; 17) rearrangement and blocks myeloid differentiation
- Epidermal growth factor receptor as a retinoid target: Epidermal growth factor receptor is often over-expressed in malignancies. Epidermal growth factor receptor activation promotes cellular growth and transformation as well as produces other biological effects. Retinoids repress epidermal growth factor receptor expression through a transcriptional mechanism [5]
- Induction of differentiation: Tretinoin and other retinoids can induce differentiation in certain malignant cell lines in mice and humans, such as acute promyelocytic leukemia, histiocytic lymphoma, neuroblastoma and embryonal carcinoma. The carboxylic acid end group may be critical for this capability of retinoids because it is crucial for binding and activation of retinoic acid receptors [6]
- Inhibition of proliferation: In addition to differentiation induction, retinoids also have a direct anti-proliferative effect which is also linked to their carboxylic acid end group
- Inhibition of ornithine decarboxylase: Ornithine decarboxylase is the rate-limiting enzyme in the synthesis of polyamines (putrescine, spermidine, and spermine). Polyamines play important roles in stabilizing DNA structure and in the DNA double-strand break repair pathway; they also function as antioxidants. Thus, ornithine decarboxylase promotes cell growth and reduces apoptosis. Ornithine decarboxylase is a transcriptional target of the oncogene Myc and is upregulated in a wide variety of cancers.[7] Inhibitors of ornithine decarboxylase such as eflornithine have been shown to effectively reduce cancers in animal models.[8] Along with their direct effect on DNA stability, polyamines also upregulate gap junction genes and downregulate tight junction genes.[9] Gap junction genes are involved in communication between carcinogenic cells and tight junction genes act as tumor suppressors.[10] Retinoids block the induction of ornithine decarboxylase by tumor promoters.
- Other possible mechanisms of action may include immunomodulation and induction of apoptosis. Physiologic doses of retinoids also stimulate killer T-cell production and cell-mediated cytotoxicity which may well be crucial in the treatment of cancer.
Efficacy of Retinoids as Chemopreventive Agents in Dermatology
The role of systemic retinoids in skin cancer prevention was first observed in xeroderma pigmentosum.[11] Shuttleworth et al. studied the efficacy of etretinate in preventing skin cancer in renal transplant recipients.[12] Although systemic retinoids are widely used in organ transplant recipients, only a few randomized controlled trials have been performed, with limitations, including small sample sizes.
In a prospective, open, randomized, cross-over trial, George et al. evaluated the efficacy of acitretin on non-melanoma skin cancer development in renal transplant recipients.[13] Acitretin (25 mg/day) was administered to 14 patients while nine patients received no therapy. Cross-over was done at 1 year. The number of squamous cell carcinomas observed in patients on acitretin was significantly lower than that in the drug-free period (P = 0.002). A similar, yet not significant, trend was observed for basal cell carcinomas. A severe rebound in squamous cell carcinoma development occurred in one patient upon discontinuation of acitretin. Poor drug tolerability resulted in a high withdrawal rate.
In a randomized, controlled, open-label trial, 26 renal transplant recipients were assigned randomly to two different 1-year treatment protocols with acitretin.[14] Thirteen patients were treated with acitretin 0.4 mg/kg/day for a year and 13 patients received acitretin 0.4 mg/kg/day during the first 3 months followed by 0.2 mg/kg/day for the remaining 9 months. In both groups, the number of actinic keratoses decreased by nearly 50% but the number of new malignant tumors during the study year was similar to the pre-treatment period. The frequency of mucocutaneous sideeffects resulted in significant dose reductions.
In a retrospective before-after study, Harwood et al. evaluated the efficacy of acitretin in the chemoprevention of squamous cell carcinomas.[15] A total of 32 organ transplant recipients received acitretin for 1–16 years. The number of squamous cell carcinomas developing annually during retinoid therapy was compared to the number of squamous cell carcinomas during the 12-month pre-treatment period. A statistically significant reduction in squamous cell carcinomas was noted in the first (P = 0.006), second (P < 0.001) and third (P = 0.02) years after starting retinoids.
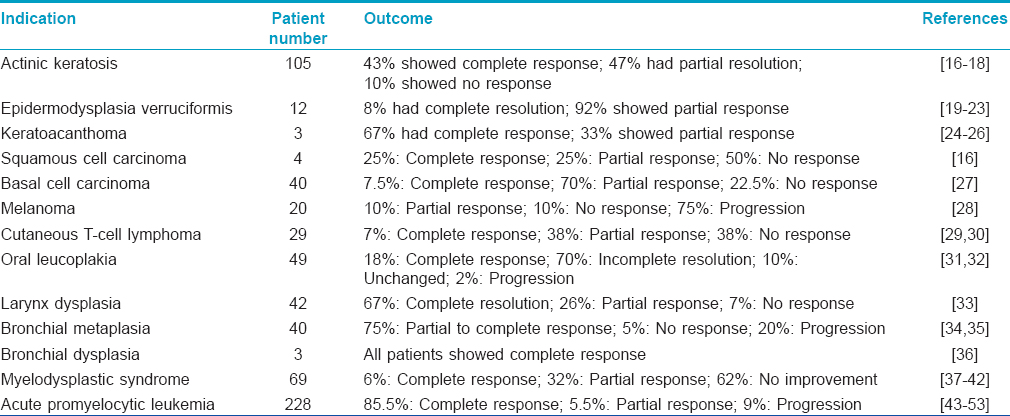
A brief summary of various other studies, highlighting the use of retinoids in cancer prevention and treatment is given in [Table - 2].
Future Prospects
Non-classical retinoids that bind nuclear retinoid X receptors (also known as rexinoids) are yet to be studied extensively in cancer chemoprevention but have been examined in certain cancer therapeutic settings. Bexarotene, a selective retinoid X receptor agonist, is an active agent in cutaneous T-cell lymphoma and was approved by the Food and Drug Administration for the treatment of this disease.[54]
Some retinoids exert their effects through retinoid receptor-independent pathways. One example is N-(4-hydroxyphenyl) retinamide that can induce apoptosis in responsive cells including those resistant to classical retinoids due to retinoid nuclear receptor defects.[55] N-(4-hydroxyphenyl) retinamide triggers its biological effects in part through the generation of reactive oxygen species. It has reported activity in the prevention of second breast cancers.
B-Rapidly Accelerated Fibrosarcoma (BRAF) inhibitors have recently been approved by Food and Drug Administration for metastatic melanoma. The main toxicity associated with these agents is the development of hyperkeratotic lesions, in particular, squamous cell carcinomas. The use of acitretin in patients on selective BRAF inhibitors is recent but it seems to be effective in reducing the number of new benign hyperkeratotic lesions as well as malignant lesions such as squamous cell carcinomas.[56]
Concerns Regarding Use of Retinoids as Chemopreventive Agents
Concerns relating to the achievement of sufficient, non-toxic levels of retinoids in target tissues are justified because of their high affinity for multiple binding sites during absorption and their self-induced metabolism enroute to target sites. Several potential strategies have been suggested to address these issues. One is the use of retinoic acid metabolism-blocking agents and a second is using locally effective formulations (e.g. aerosol formulations for the lung) of the retinoid.[57]
Despite numerous studies dealing with retinoids in chemoprevention of non-melanoma skin cancer, precise details regarding the type of retinoid to use, dosage, duration and management of side effects have not been standardized. Moreover, the use of retinoids for chemoprevention is not approved by the Food and Drug Administration (FDA), USA and hence, no guidelines exist to regulate the same.[58]
However, Hardin and Mydlarski have suggested the use of low starting doses of acitretin (i.e., 10 mg/day) for the chemoprevention of secondary malignancies in organ transplant recipients. The dose of acitretin may be increased to 30 mg/day, depending on clinical response and drug tolerability. According to them, chemoprevention should be viewed as a lifelong therapy in organ transplant recipients as rebound flare occurs upon discontinuation of retinoids.[59]
Side Effects of Retinoids
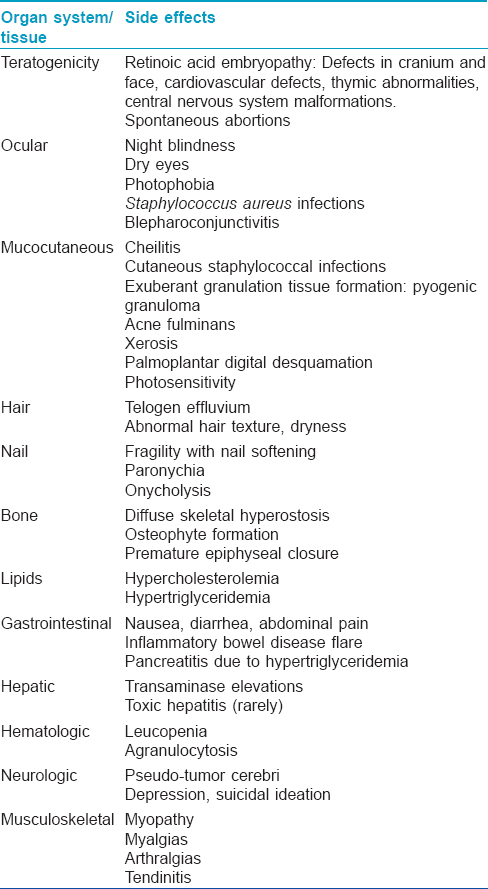
Since retinoids need to be used for long durations for prevention or treatment of skin or systemic cancers, it is important to be aware of their potential side effects. The various possible side effects are summarized in [Table - 3].
Monitoring
- Baseline: History and examination to rule out any contraindication for the use of retinoids, serum pregnancy test, complete blood count with platelets, liver function tests (aspartate aminotransferase, alanine transaminase, alkaline phosphatase, bilirubin), fasting lipid profile (triglycerides, total cholesterol, low-density lipoprotein, high-density lipoprotein cholesterol), renal function tests (blood urea nitrogen, creatinine), urinalysis (optional)
- Follow-up: Complete blood count with platelets, liver function tests, fasting lipid profile, renal function tests should be done monthly for first 3–6 months and then every 3 monthly. Serum or urine pregnancy test should be done monthly for women of childbearing potential (and at the end of treatment).
Conclusions
The development of new, more effective and less toxic retinoids, alone or in combination with other drugs, may provide additional avenues for cancer chemoprevention and therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed Proc 1976;35:1332-8.
[Google Scholar]
|
| 2. |
Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A Vitamin. J Exp Med 1925;42:753-77.
[Google Scholar]
|
| 3. |
Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, Byers RM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med 1990;323:795-801.
[Google Scholar]
|
| 4. |
Boyle JO, Langenfeld J, Lonardo F, Sekula D, Reczek P, Rusch V, et al. Cyclin D1 proteolysis: A retinoid chemoprevention signal in normal, immortalized, and transformed human bronchial epithelial cells. J Natl Cancer Inst 1999;91:373-9.
[Google Scholar]
|
| 5. |
Lonardo F, Dragnev KH, Freemantle SJ, Ma Y, Memoli N, Sekula D, et al. Evidence for the epidermal growth factor receptor as a target for lung cancer prevention. Clin Cancer Res 2002;8:54-60.
[Google Scholar]
|
| 6. |
Apfel C, Crettaz M, Siegenthaler G, Hunziker W. Synthetic retinoids: Binding to RAR. In: Saurat JH, editor. Retinoids: 10 Years on. Basel: Karger; 1991. p. 110-20.
[Google Scholar]
|
| 7. |
Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci U S A 1993;90:7804-8.
[Google Scholar]
|
| 8. |
Meyskens FL Jr, Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res 1999;5:945-51.
[Google Scholar]
|
| 9. |
Shore L, McLean P, Gilmour SK, Hodgins MB, Finbow ME. Polyamines regulate gap junction communication in connexin 43-expressing cells. Biochem J 2001;357(Pt 2):489-95.
[Google Scholar]
|
| 10. |
Gerner EW, Meyskens FL Jr. Polyamines and cancer: Old molecules, new understanding. Nat Rev Cancer 2004;4:781-92.
[Google Scholar]
|
| 11. |
DiGiovanna JJ. Retinoid chemoprevention in patients at high risk for skin cancer. Med Pediatr Oncol 2001;36:564-7.
[Google Scholar]
|
| 12. |
Shuttleworth D, Marks R, Griffin PJ, Salaman JR. Treatment of cutaneous neoplasia with etretinate in renal transplant recipients. Q J Med 1988;68:717-25.
[Google Scholar]
|
| 13. |
George R, Weightman W, Russ GR, Bannister KM, Mathew TH. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol 2002;43:269-73.
[Google Scholar]
|
| 14. |
de Sévaux RG, Smit JV, de Jong EM, van de Kerkhof PC, Hoitsma AJ. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: Clinical effects of a randomized trial comparing two doses of acitretin. J Am Acad Dermatol 2003;49:407-12.
[Google Scholar]
|
| 15. |
Harwood CA, Leedham-Green M, Leigh IM, Proby CM. Low-dose retinoids in the prevention of cutaneous squamous cell carcinomas in organ transplant recipients: a 16-year retrospective study. Arch Dermatol 2005;141:456-64.
[Google Scholar]
|
| 16. |
Grupper C, Berretti B. Cutaneous neoplasia and etretinate. In Spitzy KH, Karrer K, editors. Proceedings 13th International Congress of Chemotherapy. Vol. 11. Vienna: Egermann; 1983. p. 201/24-7.
[Google Scholar]
|
| 17. |
Moriarty M, Dunn J, Darragh A, Lambe R, Brick I. Etretinate in treatment of actinic keratosis. A double-blind crossover study. Lancet 1982;1:364-5.
[Google Scholar]
|
| 18. |
Watson AB. Preventative effect of etretinate therapy on multiple actinic keratoses. Cancer Detect Prev 1986;9:161-5.
[Google Scholar]
|
| 19. |
Edelson Y, Berretti B, Grupper C. Treatment of epidermodysplasia verruciformis or multiple verrucae planae by oral aromatic retinoid. In: Orfanos CE, Braun-Falco O, Farber EM, Grupper CL, Polano MK, Schuppli R, et al., editors. Retinoids, Advances in Basic Research and Therapy. Berlin: Springer; 1981. p. 446.
[Google Scholar]
|
| 20. |
Guilhou JJ, Malbos S, Barnéon S, Habib A, Baldet P, Meynadier J. Epidermodysplasia verruciformis (2 cases). Immunological study (author's transl). Ann Dermatol Venereol 1980;107:611-9.
[Google Scholar]
|
| 21. |
Jablonska S, Obalek S, Wolska H, Jarząbek-Chorzelska M. Ro 10-9359 in epidermodysplasia verruciformis: Preliminary report. In: Orfanos CE, Braun-Falco O, Farber EM, Grupper CL, Polano MK, Schuppli R, et al., editors. Retinoids, Advances in Basic Research and Therapy. Berlin: Springer; 1981. p. 401-5.
[Google Scholar]
|
| 22. |
Kanerva LO, Johansson E, Niemi KM, Lauharanta J, Salo OP. Epidermodysplasia verruciformis. Clinical and light- and electron-microscopic observations during etretinate therapy. Arch Dermatol Res 1985;278:153-60.
[Google Scholar]
|
| 23. |
Lutzner MA, Blanchet-Bardon C. Oral retinoid treatment of human papillomavirus type 5-induced epidermodysplasia verruciformis. N Engl J Med 1980;302:1091.
[Google Scholar]
|
| 24. |
Haydey RP, Reed ML, Dzubow LM, Shupack JL. Treatment of keratoacanthomas with oral 13-cis-retinoic acid. N Engl J Med 1980;303:560-2.
[Google Scholar]
|
| 25. |
Levine N, Miller RC, Meyskens FL Jr. Oral isotretinoin therapy. Use in a patient with multiple cutaneous squamous cell carcinomas and keratoacanthomas. Arch Dermatol 1984;120:1215-7.
[Google Scholar]
|
| 26. |
Shaw JC, White CR Jr. Treatment of multiple keratoacanthomas with oral isotretinoin. J Am Acad Dermatol 1986;15(5 Pt 2):1079-82.
[Google Scholar]
|
| 27. |
Berretti B, Grupper C, Edelson Y, Bermejo D. Aromatic retinoid in the treatment of multiple superficial basal cell carcinoma, arsenic keratoses and keratoacanthoma. In: Orfanos CE, Braun-Falco O, Farber EM, Grupper CL, Polano MK, Schuppli R, et al., editors. Retinoids, Advances in Basic Research and Therapy. Berlin: Springer; 1981. p. 397-99.
[Google Scholar]
|
| 28. |
Lippman SM, Kessler JF, Meyskens FL Jr. Retinoids as preventive and therapeutic anticancer agents (Part II). Cancer Treat Rep 1987;71:493-515.
[Google Scholar]
|
| 29. |
Claudy AL, Rouchouse B. Treatment of cutaneous T-cell lymphomas with retinoids. In Saurat JH, editor. Retinoids, New Trends in Research and Therapy. Basel: Karger; 1985. p. 335-40.
[Google Scholar]
|
| 30. |
Souteyrand P, Thivolet J, Fulton R. Treatment of parapsoriasisen plaques and mycosis fungoides with an oral aromatic retinoid (Ro 10-9359). In: Orfanos CE, Braun-Falco O, Farber EM, Grupper CL, Polano MK, Schuppli R, et al., editors. Retinoids, Advances in Basic Research and Therapy. Berlin: Springer 1981. p. 313-6.
[Google Scholar]
|
| 31. |
Koch HF. Biochemical treatment of precancerous oral lesions: The effectiveness of various analogues of retinoic acid. J Maxillofac Surg 1978;6:59-63.
[Google Scholar]
|
| 32. |
Koch HF. Effect of retinoids on precancerous lesions of oral mucosa. In: Orfanos CE, Braun-Falco O, Farber EM, Grupper CL, Polano MK, Schuppli R, et al., editors. Retinoids, Advances in Basic Research and Therapy. Berlin: Springer; 1981. p. 307-12.
[Google Scholar]
|
| 33. |
Bichler E. The role of aromatic retinoid in treatment of laryngeal keratinizing disorders and dysplasias. In: Spitzy KH, Karrer K, editors. Proceedings of the 13th International Congress of Chemotherapy. Vienna: VH Egermann, 1983. p. 201-229.
[Google Scholar]
|
| 34. |
Gouveia J, Mathé G, Hercend T, Gros F, Lemaigre G, Santelli G, et al. Degree of bronchial metaplasia in heavy smokers and its regression after treatment with a retinoid. Lancet 1982;1:710-2.
[Google Scholar]
|
| 35. |
Misset JL, Mathé G, Santelli G, Gouveia J, Homasson JP, Sudre MC, et al. Regression of bronchial epidermoid metaplasia in heavy smokers with etretinate treatment. Cancer Detect Prev 1986;9:167-70.
[Google Scholar]
|
| 36. |
Brandli O, Pedio G, Sulser H, Braumann HR. Preliminary Results of a Six Months Treatment of Bronchial Dysplasia in Heavy Smokers with Etretinate. Ill Eur Conf Clin Oncol, Stockholm; 1985. p. 22.
[Google Scholar]
|
| 37. |
Abrahm J, Besa EC, Hyzinski M, Finan J, Nowell P. Disappearance of cytogenetic abnormalities and clinical remission during therapy with 13-cis-retinoic acid in a patient with myelodysplastic syndrome: Inhibition of growth of the patient's malignant monocytoid clone. Blood 1986;67:1323-7.
[Google Scholar]
|
| 38. |
Besa EC, Hyzinski M, Nowell PC. Clinical trials and in vitro evaluation of 13-cis-retinoic acid in myelodysplastic syndrome. Proc Am Soc Clin Oncol 1985;4:216.
[Google Scholar]
|
| 39. |
Gold EJ, Mertelsmann RH, Itri LM, Gee T, Arlin Z, Kempin S, et al. Phase I clinical trial of 13-cis-retinoic acid in myelodysplastic syndromes. Cancer Treat Rep 1983;67:981-6.
[Google Scholar]
|
| 40. |
Greenberg BR, Durie BG, Barnett TC, Meyskens FL Jr. Phase I-II study of 13-cis-retinoic acid in myelodysplastic syndrome. Cancer Treat Rep 1985;69:1369-74.
[Google Scholar]
|
| 41. |
Kerndrup G, Bendix-Hansen K, Pedersen B, Ellegaard J, Hokland P. Primary myelodysplastic syndrome: Treatment of 6 patients with 13-cis retinoic acid. Scand J Haematol Suppl 1986;45:128-32.
[Google Scholar]
|
| 42. |
Picozzi VJ Jr, Swanson GF, Morgan R, Hecht F, Greenberg PL. 13-cis retinoic acid treatment for myelodysplastic syndromes. J Clin Oncol 1986;4:589-95.
[Google Scholar]
|
| 43. |
Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 1988;72:567-72.
[Google Scholar]
|
| 44. |
Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, et al. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood 1990;76:1704-9.
[Google Scholar]
|
| 45. |
Degos L, Chomienne C, Daniel MT, Berger R, Dombret H, Fenaux P, et al. Treatment of first relapse in acute promyelocytic leukaemia with all-trans retinoic acid. Lancet 1990;336:1440-1.
[Google Scholar]
|
| 46. |
Warrell RP Jr, Frankel SR, Miller WH Jr, Scheinberg DA, Itri LM, Hittelman WN, et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid). N Engl J Med 1991;324:1385-93.
[Google Scholar]
|
| 47. |
All-trans-retinoic acid followed by intensive chemotherapy gives a high complete remission rate and may prolong remissions in newly diagnosed acute promyelocytic leukemia: A pilot study on 26 cases.199280217681Fenaux P, Castaigne S, Dombret H, Archimbaud E, Duarte M, Morel P, et al. All-transretinoic acid followed by intensive chemotherapy gives a high complete remission rate and may prolong remissions in newly diagnosed acute promyelocytic leukemia: A pilot study on 26 cases. Blood 1992;80:2176-81.
[Google Scholar]
|
| 48. |
Frankel S, Weiss M, Warrell RP. A 'retinoic acid syndrome' in acute promyelocytic leukemia: Reversal by corticosteroids. Blood 1991;78:380a. [Abstract 1511].
[Google Scholar]
|
| 49. |
Chen ZX, Xue YQ, Zhang R, Tao RF, Xia XM, Li C, et al. A clinical and experimental study on all-trans retinoic acid-treated acute promyelocytic leukemia patients. Blood 1991;78:1413-9.
[Google Scholar]
|
| 50. |
Chen Z, Sun GL, Chen SJ. All-trans Retinoic Acid and Acute Promyelocytic Leukemia (APL) in China: From Clinic to Molecular Biology. Symposium: Retinoids. New Trends in Research and Clinical Applications. Palermo; 21-24 October, 1991. [Abstract 102].
[Google Scholar]
|
| 51. |
Degos L, Dombret H, Chomienne C, Daniel MT, Micléa JM, Chastang C, et al. All-trans-retinoic acid as a differentiating agent in the treatment of acute promyelocytic leukemia. Blood 1995;85:2643-53.
[Google Scholar]
|
| 52. |
Avvisati G, Petti MC, Spadea A. All-trans retinoic acid (RA) in poor risk acute promyelocytic leukemia (APL): A pilot study of the Italian cooperative group GIMEMA. Haematologica 1991;76 Suppl 4:N56.
[Google Scholar]
|
| 53. |
Ohno R, Ohshima T, Dohi H. All-trans retinoic acid as a differentiation therapy for refractory acute promyelocytic leukemia (APL). Haematologica 1991;76 Suppl 4:N152.
[Google Scholar]
|
| 54. |
Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol 2001;19:2456-71.
[Google Scholar]
|
| 55. |
Delia D, Aiello A, Lombardi L, Pelicci PG, Grignani F, Grignani F, et al. N-(4-hydroxyphenyl)retinamide induces apoptosis of malignant hemopoietic cell lines including those unresponsive to retinoic acid. Cancer Res 1993;53:6036-41.
[Google Scholar]
|
| 56. |
Anforth R, Blumetti TC, Mohd Affandi A, Fernandez-Penas P. Systemic retinoid therapy for chemoprevention of nonmelanoma skin cancer in a patient treated with vemurafenib. J Clin Oncol 2012;30:e165-7.
[Google Scholar]
|
| 57. |
Freyne E, Raeymaekers A, Venet M, Sanz G, Wouters W, De Coster R, et al. Synthesis of LIAZAL, a retinoic acid metabolism blocking agent (RAMBA) with potential clinical applications in oncology and dermatology. Bioorg Med Chem Lett 1998;8:267-72.
[Google Scholar]
|
| 58. |
Bettoli V, Zauli S, Virgili A. Retinoids in the chemoprevention of non-melanoma skin cancers: Why, when and how. J Dermatolog Treat 2013;24:235-7.
[Google Scholar]
|
| 59. |
Hardin J, Mydlarski PR. Systemic retinoids: Chemoprevention of skin cancer in transplant recipients. Skin Therapy Lett 2010;15:1-4.
[Google Scholar]
|
Fulltext Views
3,840
PDF downloads
2,587





