Translate this page into:
Risankizumab effectiveness and safety in psoriasis patients who failed other biologics: Real-life case series
Corresponding author: Ass. Prof. Dr. Noha Mohammed Dawoud, Department of Dermatology, Andrology and STDs, Faculty of Medicine, Menoufia University, Al Minufiyah, Egypt. dr_ndawoud@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Dawoud NM, El Badawy MB, Al Eid HS, Abdel Fattah MM. Risankizumab effectiveness and safety in psoriasis patients who failed other biologics: Real-life case series. Indian J Dermatol Venereol Leprol 2022;88:235-40.
Sir,
Psoriasis is a chronic relapsing immune-mediated inflammatory disease. Individuals show variable response to different biologics. Risankizumab is one of the most recently approved biologic agents for treating psoriasis which is a humanised immunoglobulin G1 monoclonal antibody that targets the p19 subunit of IL-23.1 Real-life reports regarding its effectiveness are still limited.
This study was conducted at Al Hada Armed Forces Hospital, Saudi Arabia on nine adult psoriasis patients who showed failure, intolerance and/or side effects to one or more biologic agent and were shifted to risankizumab to document its effectiveness and safety in different clinical types of psoriasis. They were prescribed risankizumab 150 mg subcutaneous injection at baseline, four weeks then every 12 weeks for at least 28 weeks. It was performed in line with the principles of the Declaration of Helsinki of 1975 (revised in 2000). Approval was granted by the regional research and ethics committee at Al Hada Armed Forces Hospital.
Clinical, demographic and treatment data were collected retrospectively from patients’ files at baseline [Table 1], four weeks of risankizumab injection and every 12 weeks thereafter. Psoriatic arthritis and its type were recorded. Severity assessment using body surface area affected, psoriasis area and severity index (PASI), physician global assessment and dermatology life quality index were analysed.
| Cases | Age (year)/sex | BMI (kg/m2) | Clinical variant | Special sites | PsA | Phototherapy | Conventional therapy | Biologics and others | Cause of biologic switch | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nail | Scalp | Genital | |||||||||
| 1 | 26/f | 21 | Plaque | - | - | - | - | + | MTX | Adalimumab Etanercept |
Failure |
| 2 | 35/f | 24 | Plaque | + | + | + | - | + | MTX | Adalimumab Etanercept |
Failure |
| 3 | 30/f | 27 | Plaque | - | + | + | - | + | MTX acitretin |
Adalimumab Etanercept |
Failure |
| 4 | 43/m | 24 | Plaque | - | - | - | Enthesitis | + | MTX acitretin |
Adalimumab Etanercept |
Failure |
| 5 | 48/m | 29 | Plaque | - | + | + | - | + | MTX acitretin |
Adalimumab Etanercept |
Failure |
| 6 | 49/f | 54.6 | Plaque | - | + | + | Peripheral joints (Small IP) | + | MTX acitretin cyclosporine |
Adalimumab Etanercept Secukinumab Ixekizumab Combination |
- Failure to anti TNF-α, - Failure to secukinumab - Severe injection site reaction to ixekizumab |
| 7 | 55/f | 34 | Plaque | - | + | - | Peripheral joints (Shoulder, hip) | _ | Adalimumab Tofacitinib |
-Failure to anti TNF- α -Failure to JAK inhibitors |
|
| 8 | 42/m | 25 | Pustular | - | MTX acitretin cyclosporine |
Adalimumab Etanercept Infliximab secukinumab |
Failure | ||||
| 9 | 37/f | 27.2 | Plaque | - | + | - | Peripheral joint (Small IP) Dactylitis | + | Etanercept | -Rising ANA titer -Limited efficacy |
|
PsA: Psoriatic arthritis, TNF: Tumour necrosis factor, MTX: Methotrexate, JAK inhibitor: Janus kinase inhibitor, IP: Interphalangeal
Following risankizumab, all measured parameters showed significant improvement over time [Figures 1a-1d]. Finally at week 28, all patients showed PASI 100 response [Figure 2]. Clinical improvement was clearly identified in various psoriasis types and localisations. Furthermore, no adverse events or relapse were reported during the study period (28 weeks) or thereafter (9-18 months follow-up period).
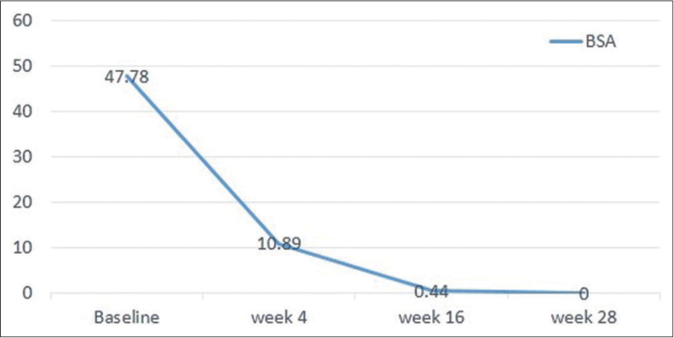
- Body surface area improvement over 28 weeks of risankizumab therapy
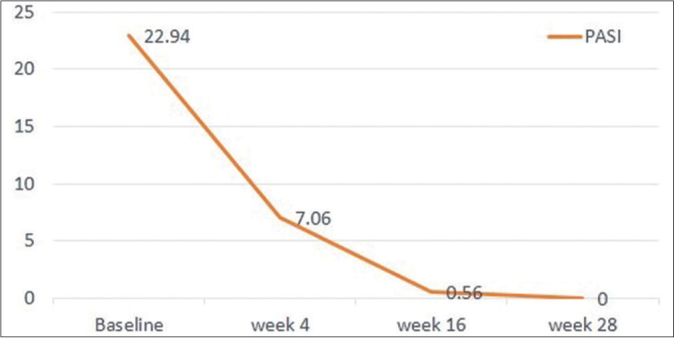
- Absolute PASI significant decline over 28 weeks of risankizumab therapy
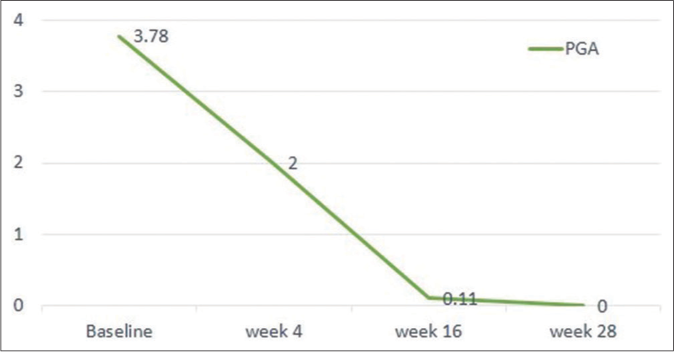
- Physician global assessment 0/1 achievement at 16/28 weeks of risankizumab therapy
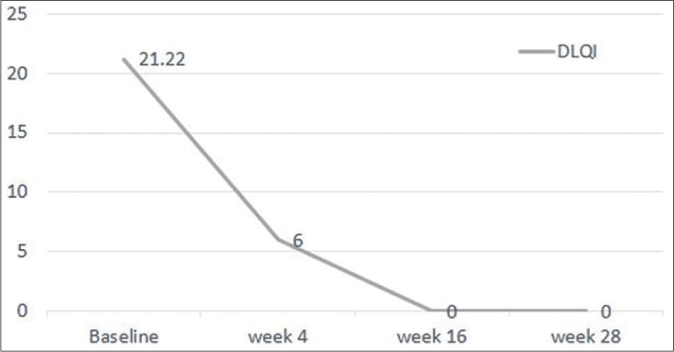
- Dermatology life quality index scores improvement over 28 weeks
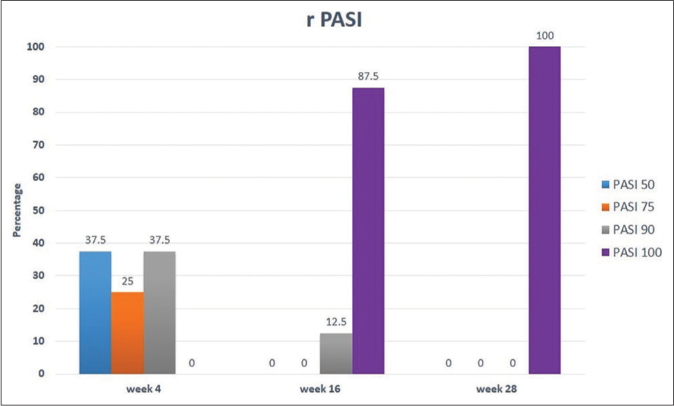
- Percentage of patients showing improvement of relative PASI throughout study time (28 weeks)
Switching therapies have become more emphasized on achieving greatest possible skin clearance (clear or almost clear), least side effects, improved quality of life and patient satisfaction. In the present study, although 90% of patients showed failure to one or more TNF-α inhibitors, they had an excellent response to risankizumab. This was in concordance with IMMvent study of Reich et al.2 who proved significant efficacy of risankizumab compared to adalimumab in achieving skin clearance for moderate-to-severe psoriasis. Furthermore, two (22.2%) patients showed failure to IL-17 antagonists following proper response on the loading dose. This was reported in IMMerge randomised clinical trial which showed that risankizumab and secukinumab efficacy was nearly similar at 16 weeks but risankizumab was superior at 52 weeks of therapy.3
In the current study, risankizumab showed excellent effectiveness among plaque psoriasis patients irrespective of their body mass index. These results are in agreement with Ruiz-Villaverde et al.1 who showed a significant decline of all scales in 100% of patients regardless of their body mass index. This was higher than what was reported in clinical trials UltIMMa-1 and U1tIMMa-2 in which results achieved PSAI 90 at week 16 in 75.3% and 74.8% of patients, respectively.4Furthermore, an Italian multicentre study showed PASI 100 improvement in 49.1% of their patients (87.5% in the present study) at week 16 with better results in females with less than 25 body mass index.5
Among our plaque psoriasis patients, 3/8 (37.5%) experienced PASI 90 response at week four which signifies rapidity of action of risankizumab. This is in agreement with Singh et al.6 meta-analysis which reported rapid response to risankizumab similar to ixekizumab and brodalumab with a better safety profile and maintenance of response. It has a faster response than guselkumab and tildrakizumab, possibly due to its stronger affinity and higher selective inhibition of the IL23-IL17 axis.
Among our patients, a morbid obese female (body mass index=54.6) [Figure 3a-c] who has shown failure to escalated doses of anti-tumour necrosis factor (TNF)-α drugs, IL-17 antagonists and combinations has demonstrated an excellent relatively fast response with PSAI75 improvement at week four [Figure 3d-f], PSAI 90 at week 16 [Figure 3g] and PASI 100 at week 28 [Figure 3h] with a sustained response up to 52 weeks. Obesity is known to be a negative prognostic marker being a risk factor to altered pharmacokinetics and higher clearance of all anti-TNF drugs, resulting in lower serum trough drug concentrations.7 This may explain why obese patients treated even with weight-based regimens such as infliximab, had lower response to treatment.8

- Baseline psoriasis lesions (abdomen) in morbid obese patient

- Baseline psoriasis lesions (back) in morbid obese patient

- Baseline psoriasis lesions (leg) in morbid obese patient

- Psoriasis lesions (right arm) after 4 weeks of risankizumab

- Psoriasis lesions (left arm) after 4 weeks of risankizumab

- Psoriasis lesions (back) after 4 weeks of risankizumab

- Psoriasis area severity index 90 response (mild erythema) at week 16 of risankizumab therapy

- Psoriasis area severity index 100 response in morbid obese female at week 28 of risankizumab therapy
Data from risankizumab Phase II and III clinical trials showed that although its blood levels are 30% lower in patients of weight >100 kg than those of patients with less weight, the levels reached are still within the therapeutic range. Hence, the usual recommended dose of risankizumab seems to remain optimal in obese patients.9
One of the presented cases had generalised pustular psoriasis. With risankizumab, the patient showed progressive improvement of body surface area affected with sustainability of that excellent response till 52 weeks. Anti-TNF-α drugs showed some efficacious results in treating pustular psoriasis, but this efficacy was dose-dependent since the majority of patients required escalating doses or reduced intervals between the doses.10 On the other hand, TNF-α inhibitors were considered as one of the drugs triggering pustular psoriasis (paradoxical reaction) and substitution of these agents by anti-p40 or anti-IL-17A may be greatly helpful.11 In Japan (March 2019), risankizumab gained its first global approval for treatment of adults with plaque psoriasis, its severe forms (pustular and erythrodermic psoriasis) and psoriatic artritis.12 Worldwide clinical trials and placebo-controlled studies investigating different ethnic groups and taking in consideration mutations of IL-36RN are recommended to examine risankizumab effectiveness in pustular psoriasis patients.
Among the studied cases, 4 (44.4%) patients suffered from psoriatic arthritis of different types with inadequate response to non-biologic disease modifying anti-rheumatic drugs, anti TNF-α, methotrexate or JAK inhibitor (tofacitinib).
Risankizumab proved to be effective in all reported types of psoriatic arthritis at the standard dose approved for skin psoriasis. The Phase III studies, KEEPsAKE-1and KEEPsAKE-2, has shown favourable clinical and radiological outcome in adult psoriatic arthritis patients treated with risankizumab versus placebo (unpublished data).12
Regarding safety, none of the patients developed any serious adverse events. Although, one of the patients had latent tuberculosis at baseline, she did not develop any symptoms of active tuberculosis till week 40 and continued safely in the study. She received prophylactic dose of once daily 300 mg isoniazid and 20 mg pyridoxine for three months, and then started risankizumab after one month of receiving the antitubercular medications. One of the patients acquired COVID-19 infection with mild symptoms denoting possible safety of risankizumab in COVID-19 patients. However, long-term safety data needs to be assessed.
Localised psoriasis were also reported; scalp in 6 (66.7%) patients, genital in 4 (44.4%) patients, and nail in one (11.1%) patient. These difficult to treat subtypes showed excellent response to risankizumab; as also stated by Dattola et al.13 However, it was noted that nail psoriasis in the form of oil drops, pitting and thickening started to recur shortly before the scheduled next dose. Further clinical studies are needed to confirm the most effective dosing for nail psoriasis.
In conclusion, risankizumab proved to be effective, safe and tolerable biologic agent for treatment of different types of psoriasis including severe form (pustular psoriasis), difficult to treat areas (nail and scalp) and psoriatic arthritis with sustainable and durable response. It might be an excellent choice for psoriasis patients who failed other biologics. However, the limited number of patients and short duration of the study make it difficult to generalise its results to all settings.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Risankizumab as a promising therapeutic approach in obese patients. Dermatol Ther. 2020;33:e13323.
- [CrossRef] [Google Scholar]
- Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): A randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-86.
- [CrossRef] [Google Scholar]
- Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): Results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-9.
- [CrossRef] [PubMed] [Google Scholar]
- Risankizumab: A pilot study of short-term effectiveness and safety in real clinical practice. Dermatol Ther. 2021;34:e14711.
- [CrossRef] [Google Scholar]
- A multicenter study on effectiveness and safety of risankizumab in psoriasis: An Italian 16-week real-life experience during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2021;35:e169-70.
- [CrossRef] [Google Scholar]
- Efficacy and safety of Risankizumab in moderate to severe psoriasis: A systematic review and meta-analysis. Dermatol Ther. 2021;34:e14487.
- [CrossRef] [Google Scholar]
- Impact of immunogenicity on pharmacokinetics, efficacy and safety of adalimumab in adult patients with moderate to severe chronic plaque psoriasis. J Eur Acad Dermatol Venereol2017;. ;31:490-7.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis. PLoS One. 2018;13:e0195123.
- [CrossRef] [PubMed] [Google Scholar]
- Exposure/ Response Relationships for Efficacy and Safety of Risankizumab in Patients with Moderate to Severe Plaque Psoriasis: Integrated Analyses of Phase 2 and 3 Clinical Trials In: Poster Presented at: The American Academy of Dermatology Annual Meeting, Washington, DC. 2019.
- [Google Scholar]
- Biologics in the treatment of pustular psoriasis. Expert Opin Drug Saf. 2020;19:969-80.
- [CrossRef] [PubMed] [Google Scholar]
- Paradoxical reactions: Anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab, and others. CurrProbl Dermatol. 2018;53:49-63.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging role of anti-IL23 in the treatment of psoriasis: When humanized is very promising. Dermatol Ther. 2020;33:e14504.
- [CrossRef] [Google Scholar]





