Translate this page into:
Risk factors for chronic and chronic-relapsing tinea corporis, tinea cruris and tinea faciei: Results of a case–control study
2 Department of Community Medicine, Government Medical College, Kannauj, Uttar Pradesh, India
Correspondence Address:
Sanjay Singh
Department of Dermatology and Venereology, Institute of Medical Sciences, Banaras Hindu University, Varanasi - 221 005, Uttar Pradesh
India
| How to cite this article: Singh S, Verma P, Chandra U, Tiwary NK. Risk factors for chronic and chronic-relapsing tinea corporis, tinea cruris and tinea faciei: Results of a case–control study. Indian J Dermatol Venereol Leprol 2019;85:197-200 |
Sir,
Over the past several years, Indian dermatologists have witnessed an unprecedented change in the epidemiology, clinical features and treatment responsiveness of tinea infections.[1],[2],[3] Recent evidence confirms the reduced effectiveness of terbinafine in this regard.[4] An evidence-based exploration of the problem will help us in identifying the modifiable risk factors resulting in chronicity and relapse of tinea infections.[1] Thus we performed a case–control study to identify the probable risk factors for chronic and chronic-relapsing tinea.
The study was conducted at Sir Sunderlal Hospital, Institute of Medical Sciences, Banaras Hindu University, Varanasi, on consecutively sampled 150 patients with chronic and chronic-relapsing tinea (see definitions below) and an equal number of age- and gender-matched controls, after obtaining permission from our Institute Ethics Committee. Patients with nail infection were excluded. Data were collected over 10 months (December 2016 to September 2017) and subsequently analyzed. Patients were consecutively selected from those attending the dermatology and venereology outpatient department on two particular days of the week. Diagnosis was confirmed in all cases by demonstrating fungal elements in potassium hydroxide microscopy. Controls were selected from normal care-givers of all dermatology patients (not only tinea patients), who accompanied them to the clinic. The controls were only included after exclusion of tinea in them. Informed consent was obtained from both groups as a mandatory pre-requisite. For each subject a case report form was filled, to provide information regarding personal data (name, date of birth, gender, weight, height, address, contact number), disease-related data (duration of illness, nature of illness (chronic or chronic-relapsing), clinical diagnosis, result of potassium hydroxide microscopy), past treatment received (steroid, antifungal, steroid/antifungal combination, steroid/antifungal/antibacterial combination or others, along with the mode of administration), information about the epidemiological variables (history of metabolic disorders including diabetes, immunodeficiency disorders, presence of excessive sweating, weekly frequency of washing clothes [undergarments, shirt/kurta/saree, trousers, jeans, towel], weekly frequency of taking bath, history of cooking food oneself, average time (minutes) spent in kitchen, children below 15 years in home, pets in home, family history of tinea, number of rooms in home and number of family members living in home).
As there is no standard definition for the term “chronic dermatophytosis,”[3] we defined chronic tinea as presence of infection for at least 3 months. Chronic-relapsing tinea was defined as presence of tinea for at least 3 months, which underwent apparent cure with treatment during this period, but relapsed within 1 month of treatment discontinuation. These definitions were formulated for this study by the authors.
Statistical analysis was performed by using the IBM SPSS Statistics software V21.0. Baseline characteristics were matched for age and gender. Continuous variables were expressed as mean and standard deviation or median and interquartile range after checking for normality. Frequencies and percentages were obtained for categorical data. Independent sample t-test or Mann–Whitney U-test was applied to compare mean and median respectively, between the cases and controls. Chi-square test was used for testing the association between the risk factors and groups. Fisher's exact test was used to test the association where expected frequency was <5. Bivariate analysis, which included all the variables studied, showed that more than one independent variables were significantly different (P < 0.05) between the patients and controls. Therefore, multivariate analysis was performed using logistic regression to understand the influence of each variable while controlling the effects of others. All P values are two-tailed.
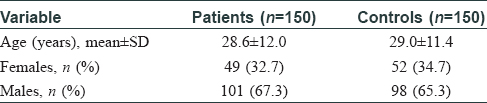
Patients and controls were similar for age and gender [Table - 1]. The patients had tinea for 6 (4.0, 9.2) months (median and interquartile range). Number of patients with tinea corporis and cruris; tinea corporis and cruris and faciei; tinea cruris; tinea corporis; tinea cruris and faciei; and tinea faciei were 72 (48.0%); 47 (31.3%); 20 (13.3%); 7 (4.7%); 3 (2.0%) and 1 (0.7%), respectively. Ninety-eight patients had chronic tinea while 52 had chronic-relapsing tinea. Sixty-three patients (42.0%) had used topical steroid in any form, while 12 (8.0%) patients had received systemic steroid before presenting to us [Table - 2].

There were some variables which were not applicable to all patients and controls. These included the use of undergarments (used by 149 of 150 patients), trousers (128 patients, 143 controls), jeans (80 patients, 94 controls), towel (139 patients, 140 controls) and time spent in kitchen (51 patients, 32 controls). Bivariate analysis showed multiple variables to be significantly different between the cases and controls [Table - 3] and [Table - 4]. Odds ratios were found to be significant for the following variables: pre-existing diabetes mellitus (unadjusted odds ratio 8.39, confidence interval 1.03–67.97, P = 0.046), family history of tinea (unadjusted odds ratio 4.62, confidence interval 2.81–7.59, P < 0.0001), personal history of cooking food (unadjusted odds ratio 2.06, confidence interval 1.22–3.48, P = 0.007), frequency of bath (days/week) (unadjusted odds ratio 0.61, confidence interval 0.44–0.84, P = 0.003), frequency of washing trousers (days/week) (unadjusted odds ratio 3.20, confidence interval 1.21–8.45, P = 0.019) and frequency of washing undergarments (days/week) (unadjusted odds ratio 0.77, confidence interval 0.65–0.90, P = 0.002).

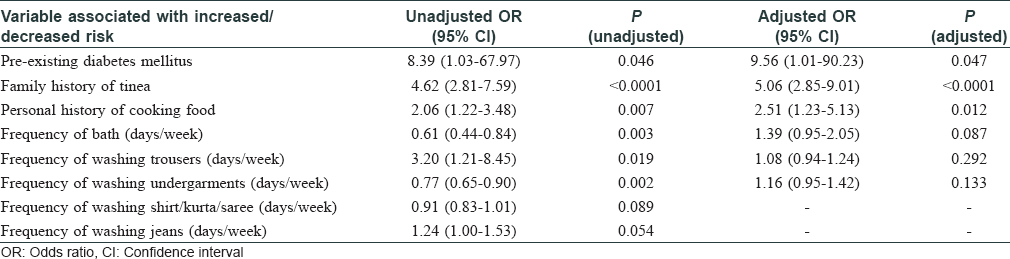
Multivariate analysis by logistic regression was performed by including those individual variables for which odds ratios were significantly different in the bivariate analysis. Variables associated with increased risk of tinea were pre-existing diabetes mellitus (adjusted odds ratio 9.56, confidence interval 1.01–90.23, P = 0.047), family history of tinea (adjusted odds ratio 5.06, confidence interval 2.85–9.01, P < 0.0001) and personal history of cooking food (adjusted odds ratio 2.51, confidence interval 1.23–5.13, P = 0.012) [Table - 4]. All patients who had diabetes were receiving treatment. We did not test their plasma glucose or glycosylated haemoglobin (HbA1c) levels.
Good internal validity of the study is attested by a strong positive association detected between chronic and chronic-relapsing tinea corporis, cruris and faciei and pre-existing diabetes mellitus, a well-known immunosuppressive condition.[5] Furthermore, the other two risk factors identified in the study, namely family history of tinea that increases risk of exposure of an individual to the pathogen and personal history of cooking food that exposes the individual to hot environment, are logically consistent. As our hospital serves patients coming from a large geographical area, most of them without being referred, we believe that the study results also have good generalisability (external validity).
We conducted a search on PubMed using key words “tinea case control” on August 11, 2018 without applying any filters. The search found 460 results, including those articles which had the words “dermatophytes” and “superficial fungal infection” in their titles, but none of them were related to case–control study on tinea corporis, tinea cruris and tinea faciei with the aim of identifying risk factors for these conditions. Therefore, in addition to having relevance for the current epidemic of “altered” dermatophytosis going on unabated in India, the results may have some relevance to dermatophytosis in general.
We did not perform a pre-study sample size calculation, but the study showed that pre-existing diabetes mellitus, family history of tinea and personal history of cooking food are risk factors for chronic and chronic-relapsing tinea corporis, tinea faciei and tinea cruris versus normal controls. It is indeed possible that a study with larger sample size may identify additional risk factors with lesser odds ratios. In the present study, we wanted to know the risk factors that make a normal individual susceptible to chronic and chronic-relapsing tinea and, therefore, normal controls were selected. If the research question is what makes a person, who had tinea, susceptible to chronicity and relapse, then the patients with tinea who were cured and did not relapse would make the appropriate control group. This would be an important area of future research. Furthermore, we do not have data on the number of sites affected in the patients, and this may be considered a limitation.
To conclude, the first data on risk factors for chronic and chronic-relapsing tinea presented here do not show that following variables increase the risk: wearing jeans, frequency of washing clothes (including undergarments, jeans and towel), frequency of taking bath, history of excessive sweating, presence of children below 15 years in home, pets in home, crowding in home and body mass index. In contrast, a positive association has been found between chronic and chronic-relapsing tinea and pre-existing diabetes mellitus, family history of tinea and personal history of cooking food. The results suggest that strategies aimed at prevention of diabetes mellitus, early treatment of a family member with tinea and restructuring of kitchen environment so that exposure to heat is prevented or minimized, are likely to decrease the chances of an individual getting chronic and chronic-relapsing tinea.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Institute of Medical Sciences, Banaras Hindu University
Conflicts of interest
There are no conflicts of interest.
| 1. |
Panda S, Verma S. The menace of dermatophytosis in India: The evidence that we need. Indian J Dermatol Venereol Leprol 2017;83:281-4.
[Google Scholar]
|
| 2. |
Verma S, Madhu R. The great Indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol 2017;62:227-36.
[Google Scholar]
|
| 3. |
Dogra S, Uprety S. The menace of chronic and recurrent dermatophytosis in India: Is the problem deeper than we perceive? Indian Dermatol Online J 2016;7:73-6.
[Google Scholar]
|
| 4. |
Singh S, Shukla P. End of the road for terbinafine? Results of a pragmatic prospective cohort study of 500 patients. Indian J Dermatol Venereol Leprol 2018;84:554-7.
[Google Scholar]
|
| 5. |
Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol 1999;26:259-65.
[Google Scholar]
|
Fulltext Views
11,913
PDF downloads
2,614





