Translate this page into:
Risk of tuberculosis with anti-tumor necrosis factor-alpha therapy in patients with psoriasis and psoriatic arthritis in Indian population
2 Department of Dermatology, KPC Medical College, Kolkata, West Bengal, India
3 Department of Dermatology, School of Medicine, Biomedical Research Institute, Pusan National University Hospital, Busan, Korea; Department of Dermatology, School of Medicine, University of California, Davis, CA, USA,
4 VA Medical Center Sacramento, Mather, CA, USA
5 Department of Critical Care Medicine, IPGME and R, Kolkata, West Bengal, India
6 Division of Rheumatology, Allergy and Clinical Immunology; Department of Dermatology, School of Medicine, University of California, Davis; VA Medical Center Sacramento, Mather, CA, USA
Correspondence Address:
Siba P Raychaudhuri
Department of Medicine and Dermatology, Division ofRheumatology, Allergy and Clinical Immunology, School of Medicine, University of California Davis, Davis, CA; VA Medical Center Sacramento, 10535 Hospital Way, Bldg #650, Research Service, Mather, CA 95655
USA
| How to cite this article: Sarkar S, Panda S, Kim B, Raychaudhuri SK, Ghosh A, Raychaudhuri SP. Risk of tuberculosis with anti-tumor necrosis factor-alpha therapy in patients with psoriasis and psoriatic arthritis in Indian population. Indian J Dermatol Venereol Leprol 2020;86:1-7 |
Abstract
Anti-tumor necrosis factor-alpha (TNF-α) immunotherapy has revolutionized the treatment of inflammatory diseases, such as psoriasis and psoriatic arthritis. However, a major concern is that patients receiving this therapy have an increased risk of infection, particularly of reactivation of latent tuberculosis (TB). There were an estimated 10.4 million new cases of tuberculosis in 2016, worldwide, and India has one of the largest TB case burden with an estimated incidence of 2.79 million cases of TB in the same year. Anti-TNF agents like etanercept and infliximab are available in India approved for psoriasis and psoriatic arthritis. But long-term use of these agents possesses a risk of reactivation of latent TB. In this review article, we assessed the risk of TB with anti-TNF therapy especially in patients with psoriasis and psoriatic arthritis in India. At the end of the article, we have also suggested a recommendation for screening of latent tuberculosis and its management, before starting anti-TNF-α therapy.
Introduction
Psoriasis affects approximately 2%–3% of world's population. The incidence of psoriasis among patients with skin disease in India ranged between 0.44% and 2.2%, with an overall incidence of 1.02%.[1] Approximately 25% of the cases are associated with psoriatic arthropathy. Psoriasis is now recognized as a systemic disease. Comorbidities include obesity, metabolic syndrome, cardiovascular, cerebrovascular, peripheral vascular disease, cardiac arrhythmia, psychiatric disorders, and psychosocial effects.[2] There are different therapeutic approaches which are already in use for the disease. Most conventional therapies available till now aim at producing clinical improvement of the disease without targeting the factors that cause psoriasis.
Biological therapies for psoriasis include drugs that are made of proteins of living organisms that target specific molecular steps important in the pathogenesis of psoriasis and psoriatic arthritis. Currently available biologicals directed against tumor necrosis factor-alpha (TNF-α) have significantly improved the treatment of psoriasis and psoriatic arthritis.[3],[4] TNF-α plays a major role in the formation of tuberculous granulomas. So, increased risks of tuberculosis (TB) are a major safety concern with anti-TNF-α treatments.[4],[5],[6],[7],[8],[9],[10],[11],[12],[13] There were an estimated 10.4 million new cases of TB disease (also known as active TB) in 2016.[14] India has one of the largest TB case burdens. The World Health Organization (WHO) TB statistics for India for 2016 gives an estimated incidence figure of 2.79 million cases of TB in India.[15] In India, anti-TNF-α are widely used by rheumatologist in the treatment of various rheumatological diseases such as rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, ulcerative colitis, and Crohn's disease. The most common anti-TNF-α agents used in India are etanercept, infliximab, and adalimumab. Although they have a long-term safety profile, these agents have been shown to increase the risk of developing TB.[16],[17],[18],[19] Etanercept and infliximab are available in India and are approved for psoriasis and psoriatic arthritis. But there are very limited data or case series of incidence of TB with the use of anti-TNF agents in patients with psoriasis and psoriatic arthritis in India. The objective of this review is to provide a comprehensive report on the risk of TB with anti-TNF-α therapy in patients with psoriasis and psoriatic arthritis in Indian population. In addition, we have also discussed evidence-based recommendation for screening and management of latent TB infection in patients with psoriasis before initiating anti-TNF-α treatment.
Search Methodologies and Outcome Information Used for Preparation of This Review
For this review, we have primarily searched English language PubMed and non-PubMed database literature to gather appropriate reports about risks of TB with anti-TNF-α therapy among patients with psoriasis/psoriatic arthritis in Indian population. For both PubMed and non-PubMed literature search, we used the following keywords: anti-TNF agents, latent TB, India, psoriasis, and psoriatic arthritis.
After reviewing articles in PubMed, we observed that Navarra et al. reported that the risk of TB is substantially higher in patients from Asia with anti-TNF-α therapy.[20] In this study, India ranked seventh out of 15 Asian countries in terms of absolute risk of TB with use of anti-TNF-α agents, and the estimated absolute risk of increase in TB (per 100,000) was 4922, 302, and 3125 respectively with adalimumab, etanercept, and infliximab therapy. In a study from India, Puri et al. reported that out of 79 patients with ulcerative colitis treated with infliximab, 3 patients developed disseminated TB and 4 developed pulmonary TB.[16] But this study did not take patients with psoriasis/psoriatic arthritis into account. Singh et al. reported a case of rectal TB after infliximab therapy despite negative screening for latent TB infection (LTBI) in a patient with ulcerative colitis.[21] We did not find any report in PubMed database about TB infection in patients with psoriasis/psoriatic arthritis with anti-TNF-α agents.
As there were no studies available in PubMed about the risk of TB infection with anti-TNF therapy in India, we broadened our PubMed search to include literature in countries where TB is endemic, and also we searched non-PubMed databases such as Google Scholar and Cochrane database. We observed that most of the studies were done on patients suffering from rheumatological disorders other than psoriasis/psoriatic arthritis. Here, we are reporting representative studies which may suggest similar possible risks of TB in Indian population. A study conducted in Spain, which is a TB endemic country, found that 42 (29%) patients were diagnosed with LTBI out of 144 patients with psoriasis. Only one patient had developed a primary active TB with infliximab therapy.[22]
In Korea, Byun et al. conducted a study among 525 patients with inflammatory bowel disease (IBD) receiving TNF-α blockers (365 TNF-α blocker-naive and 160 TNF-α blocker-exposed). The study demonstrated that the incidence of TB was significantly higher in patients with IBD receiving TNF-α blockers compared with TNF-α-blocker-naive patients (3.1% vs. 0.3%, P = 0.011); de novo pulmonary TB infection was more prevalent than reactivation of LTBI.[23]
In a Brazilian registry study, out of 1552 patients of RA, 415, 942, and 195 patients were treated with synthetic disease-modifying anti-rheumatic drugs (DMARDs), synthetic DMARDs combined with anti-TNF (etanercept, infliximab, and adalimumab), and synthetic DMARDs combined with non-anti-TNF biologics (abatacept, rituximab, and tocilizumab), respectively. The study showed that five patients developed TB (two pulmonary TB, two ganglionar TB, and one disseminated TB) in synthetic DMARDs with anti-TNF group, one pulmonary in synthetic DMARD group and no incidence of TB reported in synthetic DMARDs with non-anti-TNF biologic group.[24]
In the non-PubMed literature search, we found two more reports on anti-TNF therapy–associated TB in Indian patient population. Kumar et al. reported 7 cases of TB out of 216 patients. Out of 176 patients with infliximab therapy, 5 had reactivation of TB, and out of 40 cases of etanercept therapy, 2 had TB lymphadenitis.[19] Kumar et al. reported one case of tuberculous pleural effusion among 77 patients with spondyloarthritis after treatment with etanercept biosimilars.[25] Again, we did not find any report of TB infection in patients with psoriasis/psoriatic arthritis on treatment with anti-TNF-α agents.
Discussion
The use of anti-TNF agents has revolutionized treatment of psoriasis/psoriatic arthritis and other inflammatory diseases, but development of active TB/reactivation of LTBI after anti-TNF therapy is a matter of serious concern especially when these agents are used in TB-endemic countries.[3],[4] There are published cases of active TB infection after anti-TNF therapy in TB-endemic and non-TB-endemic countries in international literature. Except the study in Spain,[22] which included a large number of patients with psoriasis, most of the studies included patients with other rheumatological disorders. Studies on patients with psoriasis/psoriatic arthritis are still lacking globally. There is dearth of studies in India as well. Only three published articles showed active TB infection following anti-TNF therapy in Indian population, and these studies too were conducted on non-psoriatic rheumatological disorders. Studies on risk of TB with anti-TNF-α therapy in psoriasis and psoriatic arthritis in India are lacking despite India being a TB-endemic country. Anti-TNF-α agents like adalimumab, etanercept, and infliximab are approved for psoriasis/psoriatic arthritis in India. These agents showed good efficacy in treating psoriasis/psoriatic arthritis with minimal side effects, compared with conventional systemic agents. As incidence of both active TB and LTBI are high in India, and studies showed development of active TB with use of these agents, careful screening is thus crucial in patients who are about to commence anti-TNF treatment. The results of our literature search clearly send a message that as a specialist in the field of psoriatic disease we need to do ground work to identify risks for reactivation of TB following anti-TNF therapy in patients with psoriasis. We are hoping that a collaborative work with specialities like chest medicine and dermatology under the able leadership of their existing associations at national level could be an appropriate approach to address this challenge. An evidence-based recommendation for screening, assessing risk, and managing latent TB infection in patients before starting anti-TNF-α treatment is proposed in this review.
Recommendations for Latent TB Screening and Prophylaxis in Patients Planning to Be Treated With Anti-TNF Agents
There are no 100% specific or 100% sensitive methods for diagnosing latent TB infection, and with the currently available methods, one cannot predict with certainty which patients will develop active TB during anti-TNF-α therapy.
Risk Factors for Latent TB Infection
People who live in high TB incidence areas for > 3 months, those who have been in close contact with patients with infectious TB, demonstrate radiological evidence of previous TB infection, or have undergone previous treatment for active TB or LTBI are considered to be at risk for de novo TB infection or reactivation of TB following ant-TNF therapy or other immunosuppressive agents. Also, other risk factors to be considered for LTBI are poor nutritional status, elderly people, low socioeconomic status, and generalized immunosuppression from underlying conditions such as acquired immunodeficiency syndrome or simultaneous use of immune-modulating agents such as corticosteroids and chemotherapy.
Screening Strategies for Latent TB Infection
All patients should be clinically evaluated for history of chronic cough, fever of long duration, loss of appetite, weight loss, night sweats, and other clinical evidences of extrapulmonary TB (lymph nodes, genitourinary, gastrointestinal, central nervous system, bones and joints). The directives of the National Tuberculosis Program/Revised National Tuberculosis Control Program (NTP/RNTCP)[26] are targeted toward treatment of active TB. There are no definitive consensus guidelines in India on the approach to LTBI and its management. The two commonly used tests to diagnose LTBI are tuberculin skin test (TST) and interferon gamma release assay (IGRA). Both these tests detect the presence of immunological memory of T cells exposed to the mycobacteria causing TB.
TST or Purified Protein Derivative Test
The TST or Mantoux test is a tool for TB screening based on the T-cell-mediated delayed-type hypersensitivity reaction.[27],[28] A standard dose of five tuberculin units (TUs) (0.1 mL) is injected intradermally (into the skin) and read 48–72 hours later. PPD-RT 23 with Tween 80 of strength 1 TU and 2 TU are standardized tuberculins available in India supplied by the Bacillus Calmette–Guérin (BCG) Vaccine Laboratory, Guindy, Chennai. The skin induration (raised, hard area of skin and not redness) is measured by a trained healthcare professional using a ruler. A positive Mantoux test detects immune response to TB bacteria but does not identify if the disease is latent or active and also it has its limitations because of a false-positive or a false-negative response. 29 The test has a poor positive predictive value for current active disease.[30]
Interpretation of Tuberculin Reaction
Positive test
Five millimeters or more is positive in
- HIV-positive person
- Recent contacts of active TB case
- Persons with nodular or fibrotic changes on chest X-ray (CXR) consistent with old healed TB
- Organ transplant recipients and other immunosuppressed patients who are on cytotoxic immune-suppressive agents such as cyclophosphamide or methotrexate
- Patients on long term systemic corticosteroid therapy (>6 weeks) and those on a dose of prednisone ≥15 mg/day or equivalent
- End-stage renal disease.
Ten millimeters or more is positive in
- Recent arrivals (less than 5 years) from countries with high prevalence.
- Injectable drug users
- Residents and employees of high-risk congregate settings (e.g. prisons, nursing homes, hospitals, homeless shelters)
- Mycobacteriology laboratory personnel
- Persons with clinical conditions that place them at high risk (e.g. diabetes, prolonged corticosteroid therapy, leukemia, end-stage renal disease, chronic malabsorption syndromes, low body weight)
- Children less than 4 years of age, or children and adolescents exposed to adults in high-risk categories
- Infants, children, and adolescents exposed to adults in high-risk categories.
Fifteen millimeters or more is positive in
- Persons with no known risk factors for TB. Reactions larger than 15 mm are unlikely to be due to previous BCG vaccination or exposure to environmental mycobacteria.
False positive
- Infection with non-tuberculous mycobacteria
- Previous BCG vaccination
- Incorrect method of TST administration
- Incorrect interpretation of reaction
- Incorrect bottle of antigen used.
False negative
- Cutaneous anergy (anergy is the inability to react to skin tests because of a weakened immune system)
- Recent TB infection (within 8–10 weeks of exposure)
- Very old TB infection (many years)
- Very young age (less than 6 months old)
- Recent live-virus vaccination (e.g. measles and smallpox)
- Overwhelming TB disease
- Some viral illnesses (e.g. measles and chicken pox)
- Incorrect method of TST administration
- Incorrect interpretation of reaction, insufficient dose, and inadvertent subcutaneous injection.
Interferon Gamma Release Assay
When the T cells of a patient with TB are re-exposed to TB-specific antigens, they secrete IFN-γ, which can be measured by an enzyme-linked immunosorbent assay. IGRA uses the antigens of RD1 region of Mycobacteriumtuberculosis, the 6-kD early secreted antigenic target (ESAT-6), and the 10-kDa culture filtrate protein (CFP-10).[31] The IFN-γ is quantified in terms of International Units (IU)/mL and the test is positive if this value is above the upper threshold for the test. The test is considered positive if the number of cells is higher than the negative control. False-positive or false-negative results for these tests occur due to mitogen contamination of the isolated cells. Some of the major limitations are lack of reproducibility, variability due to analytical or preanalytical sources or immunological variability.[32],[33] The commercially available tests for such measurement include QuantiFERON-TB Gold assay (QFT-G), QuantiFERON-TB Gold In-Tube assay (QFTGIT), and T-SPOT TB assay. IGRAs and TST are both used for identifying LTBI, although both are unable to differentiate latent versus active TB infection. The difference between both the screening methods is given in [Table - 1].

Follow-Up Tests
According to the WHO guidelines, for a patient who tests positive on either the TST or IGRA test, a chest radiograph is mandatory to rule out the presence of active TB. If an abnormality is observed on the chest radiograph, then the patient should be treated for either active TB or LTBI depending on the clinical picture.[34] In those, with high risk of exposure to TB, even if the above-mentioned tests are negative and the CXR is normal, a contrast-enhanced computed tomography (CECT) scan of the chest may be performed to be more certain.[35],[36] A sputum examination for acid-fast bacilli staining and culture along with physical examination and medical history is a prerequisite to rule out LTBI/active TB according to the Centers for Disease Control and Prevention (CDC) guideline for primary healthcare providers to identify LTBI cases.[36]
Therapy Recommendations for Latent TB
Isoniazid therapy for a period of either 6 or 9 months is suggested by both the CDC and WHO.[27],[36] The 9-month therapy is preferred for children (2–11 years). Rifampin (rifampicin) 4-month regimen can also be prescribed as a monotherapy for LTBI treatment in patients who are intolerant to isoniazid or exposed to isoniazid-resistant TB.[37] Finally, isoniazid combination with rifamycin group (rifampicin or rifapentine as per the availability) is usually recommended in patients with exposure to infectious TB patients, or in patients who seroconvert (conversion of TST from negative to positive) or in patients showing CXR abnormalities. 36,38 Latent TB therapy recommendations and dose range are given in [Table - 2].

a Suggested Algorithm for Patients With Psoriasis and Psoriatic Arthritis: Latent TB Screening and Therapy Recommendation Prior to Anti-TNF-α Therapy
- Considering the paucity of data on anti-TNF therapy treatment in patients with psoriasis and psoriatic arthritis in India, an algorithm for TB screening, evaluation, and treatment is recommended [Figure - 1].
- The patient's medical history, BCG vaccination status, and previous exposure to TB should be recorded. In patients with clinical signs and symptoms of pulmonary TB and/or extrapulmonary TB, appropriate laboratory and imaging studies need to be done for confirmation of active TB infection. Patients with active TB are to be treated with appropriate anti-TB medications.
- In the absence of history of TB, a TST can be performed in BCG non-vaccinated individuals or IGRA can be performed in BCG-vaccinated individuals. For immunocompromised patients and patients on immunosuppressive therapies, IGRA is recommended.[39],[40] If positive, LTBI is to be suspected. The testing doses of the PPD need to be standardized based on the patient history. Additionally, different cut-off levels of induration with TST are suggested based on the history, risk factors, and contact with TB cases. TST sensitivity in immunocompromised patients, malnourished patients, or patients on steroid therapy may be increased by using higher concentrations of PPD for the test (10 TU PPD instead of 1 TU PPD) so as to reduce the chances of a false-negative result.[41]
- After this screening, a CXR is proposed. If the radiograph shows old scar or doubtful lesion, to verify it further, a CECT scan can be done to see active TB lesion, if any. In case of normal CXR and normal CECT chest, it should be treated as LTBI. In case of normal CXR and lesions suggestive of active TB in CECT chest, then it should be treated for active TB. At all stages, it is important to rule out the presence of active TB. If active TB is detected, then the patient should be prescribed therapy in line with RNTCP recommendations.
- For prophylaxis, isoniazid 6–9 months may be prescribed. The necessity for continuation of the therapy after the end of treatment is advised on a case-to -case basis.[42]
- If the clinical picture demands, anti-TNF therapy may be started after 1–2 months of prophylaxis.[43] In some cases, the physician may decide to initiate anti-TNF/biologic therapy only after the TB prophylaxis is complete.
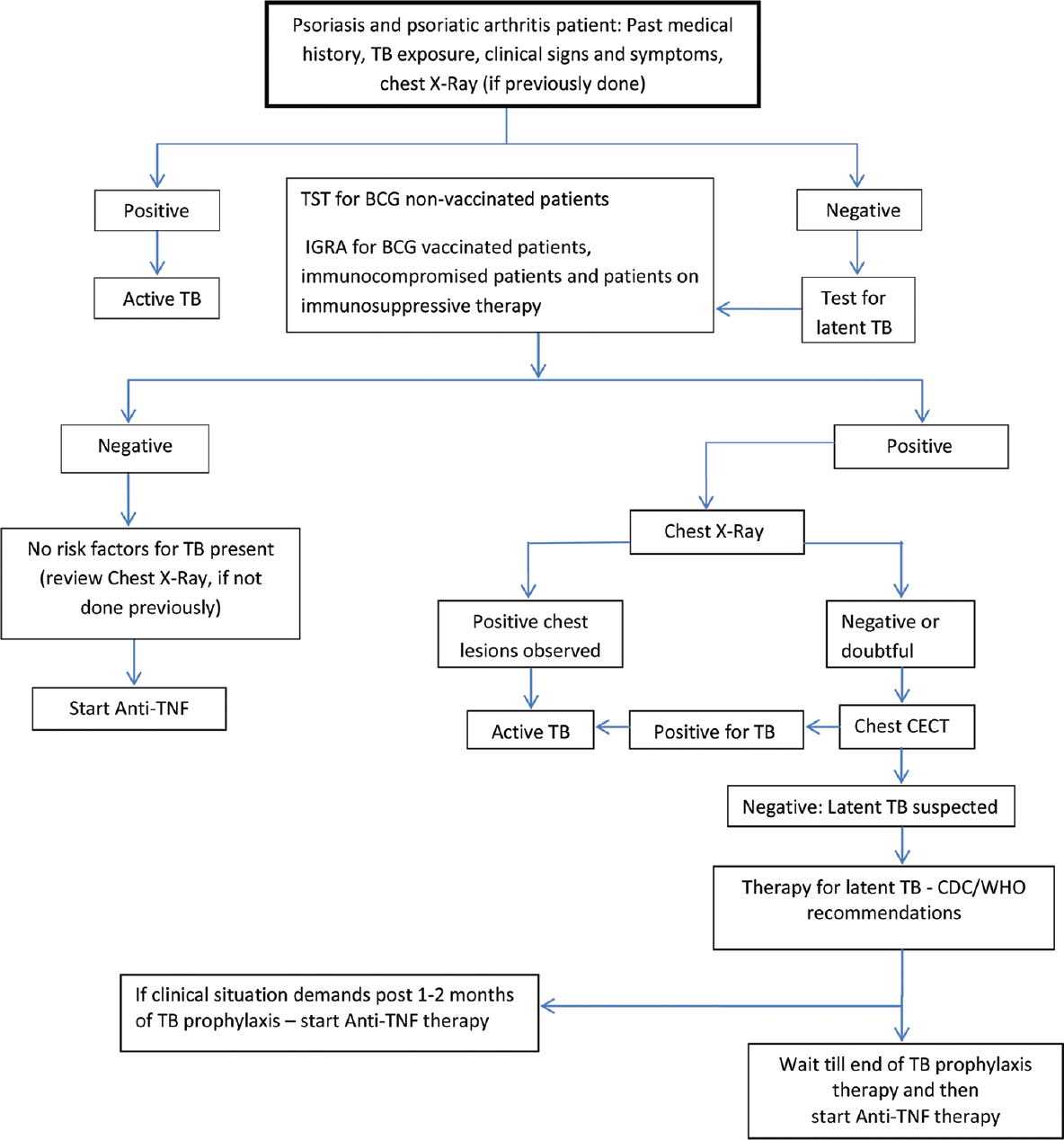 |
| Figure 1: Recommendation: screening and treatment algorithm for latent tuberculosis infection flare risk in patients with psoriasis and psoriatic arthritis in India |
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Okhandiar RP, Banerjee BN. Psoriasis in the tropics: An epidemiological survey. J Indian Med Assoc 1963;41:550-6.
[Google Scholar]
|
| 2. |
Raychaudhuri SP. Comorbidities of psoriatic arthritis – Metabolic syndrome and prevention: A report from the GRAPPA 2010 annual meeting. J Rheumatol 2012;39:437-40.
[Google Scholar]
|
| 3. |
Raychaudhuri SP, Wilken R, Sukhov AC, Raychaudhuri SK, Maverakis E. Management of psoriatic arthritis: Early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun 2017;76:21-37.
[Google Scholar]
|
| 4. |
Raychaudhuri SP, Nguyen CT, Raychaudhuri SK, Gershwin ME. Incidence and nature of infectious disease in patients treated with anti-TNF agents. Autoimmun Rev 2009;9:67-81.
[Google Scholar]
|
| 5. |
Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Cöster L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum 2005;52:1986-92.
[Google Scholar]
|
| 6. |
Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: Results from the British Society for Rheumatology biologics register. Arthritis Rheum 2006;54:2368-76.
[Google Scholar]
|
| 7. |
Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor alpha therapy. Arthritis Rheum 2003;48:3013-22.
[Google Scholar]
|
| 8. |
Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD; BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: A multicenter active-surveillance report. Arthritis Rheum 2003;48:2122-7.
[Google Scholar]
|
| 9. |
Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001;345:1098-104.
[Google Scholar]
|
| 10. |
Seong SS, Choi CB, Woo JH, Bae KW, Joung CL, Uhm WS, et al. Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): Effects of RA itself and of tumor necrosis factor blockers. J Rheumatol 2007;34:706-11.
[Google Scholar]
|
| 11. |
Wolfe F, Michaud K, Anderson J, Urbansky K. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum 2004;50:372-9.
[Google Scholar]
|
| 12. |
Mohan AK, Coté TR, Block JA, Manadan AM, Siegel JN, Braun MM. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. Clin Infect Dis 2004;39:295-9.
[Google Scholar]
|
| 13. |
Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 2004;38:1261-5.
[Google Scholar]
|
| 14. |
Global Tuberculosis report 2017 - World Health Organization. Available from: https://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf. [Last accessed on 2018 Jun 20].
[Google Scholar]
|
| 15. |
TB India 2017: Revised National TB Control Programme Annual Status Report. Available from: http://www.tbonline.info/posts/2017/6/4/tb-india-2017-rntcp-annual-status-report/. [Last accessed on 2018 Sep 01].
[Google Scholar]
|
| 16. |
Puri AS, Desai D, Sood A, Sachdeva S. Infliximab-induced tuberculosis in patients with UC: Experience from India – A country with high prevalence of tuberculosis. J Gastroenterol Hepatol 2017;32:1191-4.
[Google Scholar]
|
| 17. |
Grover R, Kapoor S, Marwaha V, Malaviya AN, Gupta R, Kumar A. Clinical experience with infliximab in spondyloarthropathy: An open label study on fourteen patients. J Indian Rheumatol Assoc 2005;13:78-82.
[Google Scholar]
|
| 18. |
Narayanan K, Shanmuganandan KG. Safety and efficacy profile of infliximab in inflammatory arthritis. Indian Rheumatism Assoc 2003: 11:1 (Abstract).
[Google Scholar]
|
| 19. |
Kumar A. Experience with anti-tumor necrosis factor-α therapy in India. APLAR J Rheumatol 2006;9:136-41.
[Google Scholar]
|
| 20. |
Navarra SV, Tang B, Lu L, Lin HY, Mok CC, Asavatanabodee P, et al. Risk of tuberculosis with anti-tumor necrosis factor-α therapy: Substantially higher number of patients at risk in Asia. Int J Rheumatic Dis 2014;17:291-8.
[Google Scholar]
|
| 21. |
Singh J, Puri AS, Sachdeva S, Sakhuja P, Arivarasan K. Rectal tuberculosis after infliximab therapy despite negative screening for latent tuberculosis in a patient with ulcerative colitis. Intest Res 2016;14:183-6.
[Google Scholar]
|
| 22. |
Sánchez-Moya AI, Dauden E. Incidence of tuberculosis infection in psoriatic patients on anti-TNF therapy: Report of a case series with 144 patients. J Eur Acad Dermatol Venereol 2011;25:730-3.
[Google Scholar]
|
| 23. |
Byun JM, Lee CK, Rhee SY, Kim HJ, Kim JW, Shim JJ, et al. The risk of tuberculosis in Korean patients with inflammatory bowel disease receiving tumor necrosis factor-α blockers. J Korean Med Sci 2015;30:173-9.
[Google Scholar]
|
| 24. |
Yonekura CL, Oliveira RD, Titton DC, Ranza R, Ranzolin A, Hayata AL, et al. Incidence of tuberculosis among patients with rheumatoid arthritis using TNF blockers in Brazil: Data from the Brazilian registry of biological therapies in rheumatic diseases (Registro Brasileiro de Monitoração de Terapias Biológicas – Biobadabrasil). Rev Bras Reumatol Engl Ed 2017;57 Suppl 2:477-83.
[Google Scholar]
|
| 25. |
Kumar A, Goel A, Lapsiwala M, Goyal M, Dembla G. Clinical experience with two etanercept biosimilars in Indian patients with spondyloarthritis. Indian J Rheumatol 2017;12:139.
[Google Scholar]
|
| 26. |
TB India 2016. Revised National TB Control Program. Annual Status Report. Available from: https://tbcindia.gov.in/index1. [Last accessed on 2018 Jun 20].
[Google Scholar]
|
| 27. |
World Health Organisation. WHO Tuberculosis. Guidelines on the Management of Latent Tuberculosis Infection. World Health Organisation; 2015. Available from: https://www.who.int/tb/publications/latent-tuberculosis-infection/en/. [Last accessed on 2018 Jul 20].
[Google Scholar]
|
| 28. |
Ayub A, Yale SH, Reed KD, Nasser RM, Gilbert SR. Testing for latent tuberculosis. Clin Med Res 2004;2:191-4.
[Google Scholar]
|
| 29. |
Nayak S, Acharjya B. Mantoux test and its interpretation. Indian Dermatol Online J 2012;3:2-6.
[Google Scholar]
|
| 30. |
Druszczynska M, Wlodarczyk M, Kielnierowski G, Seweryn M, Wawrocki S, Rudnicka W. CD14-159C/T polymorphism in the development of delayed skin hypersensitivity to tuberculin. PLoS One 2017;12:e0190106.
[Google Scholar]
|
| 31. |
Teutschbein J, Schumann G, Möllmann U, Grabley S, Cole ST, Munder T. A protein linkage map of the ESAT-6 secretion system 1 (ESX-1) of Mycobacterium tuberculosis. Microbiol Res 2009;164:253-9.
[Google Scholar]
|
| 32. |
Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014;27:3-20.
[Google Scholar]
|
| 33. |
Diel R, Nienhaus A, Lange C, Meywald-Walter K, Forssbohm M, Schaberg T. Tuberculosis contact investigation with a new, specific blood test in a low-incidence population containing a high proportion of BCG-vaccinated persons. Respir Res 2006;7:77.
[Google Scholar]
|
| 34. |
Raniga S, Parikh N, Arora A, Vaghani M, Vora PA, Vaidya V. Is HRCT reliable in determining disease activity in pulmonary tuberculosis? Indian J Radiol Imaging 2006;16:221.
[Google Scholar]
|
| 35. |
Bhalla AS, Goyal A, Guleria R, Gupta AK. Chest tuberculosis: Radiological review and imaging recommendations. Indian J Radiol Imaging 2015;25:213-25.
[Google Scholar]
|
| 36. |
CDC Latent TB Treatment. Treatment for Latent TB Infection. Available from: http://www.cdc.gov/tb/topic/treatment/ltbi.htm. [Last accessed on 2018 Aug 15].
[Google Scholar]
|
| 37. |
Center for Disease Control and Prevention. Latent Tuberculosis Infection: A Guide for Primary Health Care Providers. Atlanta, USA: Center for Disease Control and Prevention; 2014. Available from: https://www.cdc.gov/tb/publications/ltbi/default.htm. [Last accessed on 2018 Aug 12].
[Google Scholar]
|
| 38. |
Sharma SK, Sharma A, Kadhiravan T, Tharyan P. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB. Evid Based Child Health 2014;9:169-294.
[Google Scholar]
|
| 39. |
Rajagopalan M, Mital A. Biologics use in Indian psoriasis patients. Indian Dermatol Online J 2016;7:489-97.
[Google Scholar]
|
| 40. |
Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA, et al. British Association of Dermatologists' guidelines for biologic interventions for psoriasis 2009. Br J Dermatol 2009;161:987-1019.
[Google Scholar]
|
| 41. |
Phadke MA, Kshirsagar NA. Blood tests for diagnosis of tuberculosis. Lancet 2006;368:282.
[Google Scholar]
|
| 42. |
Malaviya AN, Kapoor S, Garg S, Rawat R, Shankar S, Nagpal S, et al. Preventing tuberculosis flare in patients with inflammatory rheumatic diseases receiving tumor necrosis factor-alpha inhibitors in India – An audit report. J Rheumatol 2009;36:1414-20.
[Google Scholar]
|
| 43. |
Handa R, Misra R, Chaturvedi VP, Pispati PK, Rao UR, Joshi VR; Indian Rheumatology Association. Guidelines for tuberculosis prophylaxis during anti-tumour necrosis factor-α treatment: Indian Rheumatology Association. APLAR J Rheumatol 2006;9:181-3.
[Google Scholar]
|
Fulltext Views
7,694
PDF downloads
3,688





