Translate this page into:
Risk of venous thromboembolism in patients with bullous pemphigoid: A systematic review and meta-analysis
2 Department of Internal Medicine, Bassett Medical Center, Cooperstown, NY 13326, USA
Correspondence Address:
Patompong Ungprasert
Department of Internal Medicine, Division of Rheumatology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA
| How to cite this article: Ungprasert P, Wijarnpreecha K, Thongprayoon C. Risk of venous thromboembolism in patients with bullous pemphigoid: A systematic review and meta-analysis. Indian J Dermatol Venereol Leprol 2018;84:22-26 |
Abstract
Background and Objectives: Increased risk of venous thromboembolism is observed in several autoimmune inflammatory disorders. However, data on bullous pemphigoid, one of the most common autoimmune blistering disorders, is limited. This systematic review and meta-analysis was conducted to summarize all available evidence.Methods: Two investigators independently searched published studies indexed in MEDLINE and EMBASE from inception to July 2016 using the terms for bullous pemphigoid and venous thromboembolism. The inclusion criteria were as follows: (1) cohort or case-control study evaluated the association between bullous pemphigoid and risk of venous thromboembolism, (2) effect estimates were provided as odds ratios, relative risk, hazard ratio, standardized incidence ratio with 95% confidence intervals, and (3) subjects without bullous pemphigoid were used as comparators for cohort studies, while subjects without venous thromboembolism were used as comparators for case-control studies. Point estimates and 95% confidence intervals were extracted from each study and were pooled together using the random-effect model, generic inverse variance method of DerSimonian and Laird. Cochran's Q test and the I2 statistic were used to evaluate the statistical heterogeneity.
Results: Two retrospective cohort studies, one prospective cohort study, and one case-control study met the eligibility criteria and were included in the meta-analysis. The pooled odds ratio was 2.69 (95% confidence interval, 1.79–4.05). Statistical heterogeneity was high with I2 of 77%.
Limitation: Limited accuracy of diagnosis of primary studies and high between-study heterogeneity.
Conclusion: This meta-analysis demonstrated that patients with bullous pemphigoid have a significantly increased risk of venous thromboembolism.
Introduction
Deep venous thrombosis and pulmonary embolism, collectively known as venous thromboembolism, are common medical conditions with an estimated incidence of 1–2 incident cases per 1,000 population per year.[1],[2],[3] Despite advances in diagnosis and treatment, patients with venous thromboembolism continue to have significant morbidity and mortality, with a one year all-cause mortality as high as 24 percent.[4] Traditional risk factors for venous thromboembolism include cancer, hospitalization, surgery, trauma, aging, inherited hypercoagulability, and use of hormonal therapy including oral contraceptive pills.[5],[6]
Over the past years, chronic inflammation has been increasingly recognized as a non-traditional risk factor of venous thromboembolism. Several epidemiologic studies have revealed an increased risk of both deep venous thrombosis and pulmonary embolism among patients with autoimmune disorders, such as systemic lupus erythematosus, psoriasis, rheumatoid arthritis, and systemic vasculitis.[7],[8],[9],[10],[11] The ability of inflammatory cytokines to stimulate the coagulation cascade appears to play a major role in the predisposition towards thrombogenesis among these patients.[12]
Bullous pemphigoid (BP) is defined as an autoimmune blistering disorder, characterized by the presence of autoantibodies binding against two specific structural proteins of the dermal-epidermal junction including bullous pemphigoid antigen 180 (BP180) and bullous pemphigoid antigen 230 (BP 230).[13] Patients with BP usually present with urticarial erythema and tense blisters on the trunk and extremities. Mucosal involvement is observed in 10–20% of cases. The incidence of bullous pemphigoid ranges from 5 to 60 new cases per million per year with the peak incidence observed in the seventh and eighth decade of life.[14]
Similar to other autoimmune inflammatory disorders, BP may increase the risk of venous thromboembolism. However, epidemiological data on this association is still limited. This systematic review and meta-analysis was conducted with the attempt to summarize all available studies that compared the venous thromboembolism risk in patients with bullous pemphigoid versus subjects without bullous pemphigoid.
Methods
Search strategy
All investigators independently searched published studies indexed in MEDLINE and EMBASE from inception to July 2016 using the search strategy that comprised of terms for venous thromboembolism and bullous pemphigoid as described in the [Supplementary Material 1 [SUPPORTING:1]]. No language limitation was applied. Bibliography of included studies and selected review articles were also manually searched for additional studies.
Inclusion criteria
Studies were included in this meta-analysis if they met the following inclusion criteria: (1) cohort (either prospective or retrospective) or case-control study that evaluated the association between BP and risk of venous thromboembolism, (2) effect estimates were provided as odds ratios, relative risk, hazard ratio, standardized incidence ratio with 95% confidence intervals, and (3) subjects without BP were used as comparators for cohort studies, while subjects without venous thromboembolism were used as comparators for case-control studies.
Study eligibility was independently evaluated by the two investigators (P.U. and K.W.). The third investigator (C.T.) served as the deciding vote when disagreement between the first two investigators arose. Newcastle-Ottawa scale was used to evaluate the methodological quality of the included studies.[15] This scale assessed the study in three areas including: (1) the representativeness of the subjects, (2) the comparability between the study groups, and (3) the quality of methods used to ascertain the exposure of interest for case-control studies and the outcome of interest for cohort studies.
Data extraction
The two investigators independently extracted the following data from each study: title of the article, first author's name, year of publication, year when the study was conducted, country of origin, methods used to identify bullous pemphigoid and venous thromboembolism, number of subjects in each group, demographic data of the subjects (age, sex, and ethnicity), confounders that were assessed and adjusted effect estimates with 95% confidence intervals s from multivariate model. A standardized data collection form was used to facilitate this process.
Statistical analysis
Point estimates and 95% confidence intervals were extracted from each study and were pooled together using the random-effect model, generic inverse variance method described by DerSimonian and Laird.[16] We elected not to use fixed-effect model in view of possible high inter- study variance. As the outcome of interest was relatively uncommon, relative risk and hazard ratio of cohort studies was used as an estimate to combine with odds ratio of case-control studies. Cochran's Q test, which is complemented with the I2 statistic, was used to evaluate the statistical heterogeneity. This I2 statistic quantifies the proportion of the total variation across studies that is due to true heterogeneity rather than chance. A value of I2 of 0% to 25% represents insignificant heterogeneity, >25% but <50% represents low heterogeneity, >50% but <75% represents moderate heterogeneity, and >75% represents high heterogeneity.[17] We planned to assess for publication bias by visualizing the funnel plots. All statistical analyses were conducted using Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom).
Results
The search strategy yielded 124 potentially relevant articles (9 articles from Medline and 115 articles from EMBASE). After the exclusion of 9 duplicated articles, 115 of them underwent title and abstract review. Ninety-five articles were excluded at this stage as they clearly did not meet inclusion criteria based on the type of article (they were case reports, correspondences, review articles, basic science/animal studies or randomized controlled trials), leaving 20 articles for full-length article review. After full-length review, 16 articles were excluded because they were descriptive studies without comparators or did not report the outcome of interest (venous thromboembolism). Four articles met the inclusion criteria and were included in the meta-analysis.[18],[19],[20],[21] Manual review of the included studies and some selected review articles did not yield additional eligible studies. [Figure - 1] summarizes the literature review and identification process. The main characteristics and the Newcastle-Ottawa scores of the included studies are summarized in [Table - 1].
 |
| Figure 1: Literature review and study selection process |

The included articles consisted of two retrospective cohort studies, one prospective cohort study, and one case-control study. All studies were conducted in Europe (England, Italy, and Denmark). All but one study were medical registry-based studies that relied on diagnostic codes for identification and verification of the events of interest.
This meta-analysis found a significantly increased risk of venous thromboembolism among patients with bullous pemphigoid with the pooled odds ratio from the four studies of 2.69 (95% confidence interval, 1.79–4.05). However, the statistical heterogeneity was high with I2 of 77%. [Figure - 2] demonstrates the forest plot of this meta-analysis.
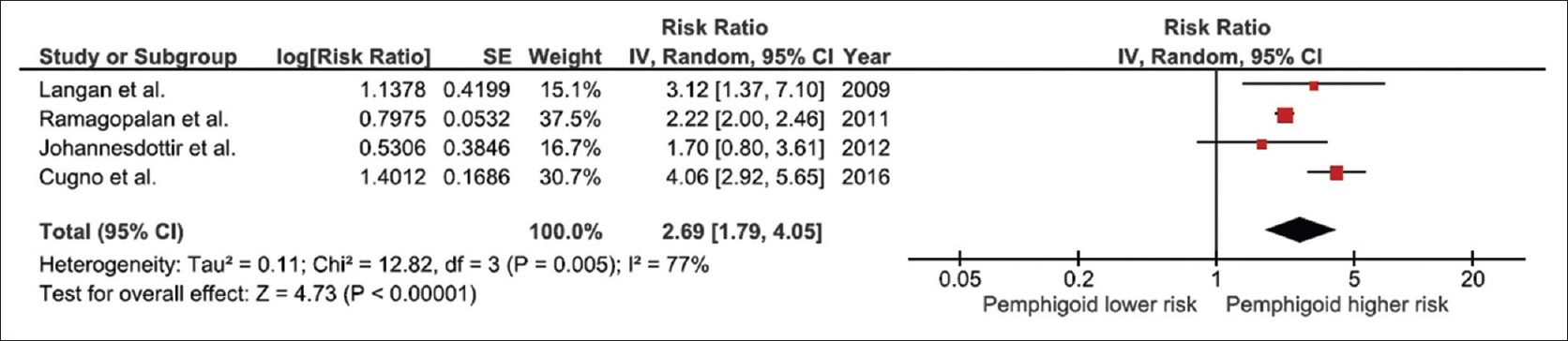 |
| Figure 2: Forest plot of all included studies |
Sensitivity analysis
To confirm the robustness of the pooled results, jack-knife sensitivity analysis, which excluded one study from the meta-analysis at a time was performed. The pooled odds ratio from this sensitivity analysis changed slightly from the complete analysis, ranged from 2.22 to 3.01 and remained statistically significant. It should be noted that the pooled sensitivity analysis, excluding the only case-control study by Johannesdottir et al.,[20] was 2.96 (95% confidence interval, 1.83–4.80), which was not significantly different from the complete analysis.
Interestingly, the I2 of the sensitivity analysis that excluded the study by Cugno et al.[21] was zero, indicating that this study was the major source of the between study variance. Nonetheless, the pooled effect estimate of the sensitivity analysis excluding the study by Cugno et al. was not significantly different from the complete analysis with the pooled odds ratio of 2.22 (95% confidence interval, 2.00–2.46).
Evaluation for publication bias
Evaluation for publication bias by visualization of funnel plot was not performed as only four studies were included in this meta-analysis, which would prevent meaningful interpretation of the plot.
Discussion
The current study is the first systematic review and meta-analysis that summarizes all available studies on the association between venous thromboembolism and bullous pemphigoid. We found a 2.69-fold increased risk of venous thromboembolism among patients with bullous pemphigoid. The results are in line with previous studies that observed approximately 1.5 to 4-fold increased risk of venous thromboembolism among patients with systemic autoimmune disorders.[19],[22]
Chronic inflammation is the common feature of systemic autoimmune disorders and therefore most likely the key element in perpetuating endothelial dysfunction and promoting a thrombophilic state among these conditions. Specific mechanisms by which this occurs in each autoimmune disease may vary based on their predominant molecular pathogenesis. However, common underlying mechanisms of inflammation-induced thrombosis include upregulation of coagulation factors, downregulation of anticoagulants, and decreased fibrinolysis by inflammatory cytokines as well as injury to endothelial cells by oxidative stress.[12],[23] Further support that inflammation plays a key role in the development of venous thromboembolism is the higher proportion of events occurring at the time of diagnosis or soon after diagnosis of autoimmune diseases,[22],[24],[25] as well as the higher incidence of venous thromboembolism with active disease compared with inactive disease.[26],[27]
The increased risk of venous thromboembolism among patients with bullous pemphigoid may have implications on clinical practice. Physicians should have a higher index of suspicion for deep venous thrombosis/pulmonary embolism in patients with bullous pemphigoid who present with leg swelling, leg pain, or respiratory symptoms. Prophylactic anticoagulation may be indicated in patients with bullous pemphigoid, particularly those with other traditional venous thromboembolism risk factors such as malignancy or when they were hospitalized. However, further prospective studies are required to determine the role of venous thromboembolism prevention among these patients.
Limitations
Even though the literature review process was rigorous and the quality of the included studies was high as reflected in the high Newcastle-Ottawa scores, we acknowledge that there are some limitations. Firstly, most of the included studies were medical registry-based studies, with the exception of the study by Cugno et al.[21], which are inherently at risk of inaccurate and incomplete coding for both bullous pemphigoid and venous thromboembolism. Secondly, statistical heterogeneity was high in this study. Interestingly, the I2 dropped dramatically to 0% with the sensitivity analysis that excluded the study by Cugno et al.[21] We suspect that the difference in study methodology was responsible for this high heterogeneity, as Cugno et al.'s study was the only one that was not medical registry based, and confirmed the diagnosis of BP and venous thromboembolism by office visits and medical record reviews. However, the pooled odds ratio of the sensitivity analysis, excluding the study by Cugno et al., was not significantly different from the complete analysis. Similarly, exclusion of the only case-control study[20] did not significantly alter the pooled result. Thirdly, this is a meta-analysis of observational studies that can only demonstrate an association but cannot determine causality. It is possible that confounders that were not adjusted in the primary studies, rather than bullous pemphigoid itself, are responsible for the increased tendency of venous thromboembolism. In particular, none of the included studies adjusted their effect estimates for glucocorticoid exposure, a known risk factor for venous thromboembolism.[28] Lastly, surveillance bias may also play a role. It is possible that patients with BP may have more investigations performed on them, including imaging studies because they have more visits to medical providers because of their chronic illness. Therefore, interpretation of the findings of this meta-analysis needs to be performed with caution.
Conclusion
In conclusion, this meta-analysis demonstrated a statistically significant increased venous thromboembolism risk among patients with bullous pemphigoid. How this risk should be addressed in clinical practice remains unclear and requires further investigation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: A population-based study. J Thromb Haemost 2007;5:692-9.
[Google Scholar]
|
| 2. |
Anderson FA Jr., Zayaruzny M, Heit JA, Fidan D, Cohen AT. Estimated annual numbers of US acute-care hospital patients at risk for venous thromboembolism. Am J Hematol 2007;82:777-82.
[Google Scholar]
|
| 3. |
Liao S, Woulfe T, Hyder S, Merriman E, Simpson D, Chunilal S. Incidence of venous thromboembolism in different ethnic groups: A regional direct comparison study. J Thromb Haemost 2014;12:214-9.
[Google Scholar]
|
| 4. |
Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, et al. The clinical course of pulmonary embolism. N Engl J Med 1992;326:1240-5.
[Google Scholar]
|
| 5. |
Hawbaker S. Venous thromboembolism in the cancer population: Pathology, risk, and prevention. J Adv Pract Oncol 2012;3:23-33.
[Google Scholar]
|
| 6. |
Sidney S, Cheetham TC, Connell FA, Ouellet-Hellstrom R, Graham DJ, Davis D, et al. Recent combined hormonal contraceptives (CHCs) and the risk of thromboembolism and other cardiovascular events in new users. Contraception 2013;87:93-100.
[Google Scholar]
|
| 7. |
Aviña-Zubieta JA, Bhole VM, Amiri N, Sayre EC, Choi HK. The risk of deep venous thrombosis and pulmonary embolism in giant cell arteritis: A general population-based study. Ann Rheum Dis 2016;75:148-54.
[Google Scholar]
|
| 8. |
Bazzan M, Vaccarino A, Marletto F. Systemic lupus erythematosus and thrombosis. Thromb J 2015;13:16.
[Google Scholar]
|
| 9. |
Ungprasert P, Sanguankeo A, Upala S, Suksaranjit P. Psoriasis and risk of venous thromboembolism: A systematic review and meta-analysis. QJM 2014;107:793-7.
[Google Scholar]
|
| 10. |
Ungprasert P, Srivali N, Spanuchart I, Thongprayoon C, Knight EL. Risk of venous thromboembolism in patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol 2014;33:297-304.
[Google Scholar]
|
| 11. |
Koutroumpakis EI, Tsiolakidou G, Koutroubakis IE. Risk of venous thromboembolism in patients with inflammatory bowel disease. Semin Thromb Hemost 2013;39:461-8.
[Google Scholar]
|
| 12. |
Nagareddy P, Smyth SS. Inflammation and thrombosis in cardiovascular disease. Curr Opin Hematol 2013;20:457-63.
[Google Scholar]
|
| 13. |
Lo Schiavo A, Ruocco E, Brancaccio G, Caccavale S, Ruocco V, Wolf R. Bullous pemphigoid: Etiology, pathogenesis, and inducing factors: Facts and controversies. Clin Dermatol 2013;31:391-9.
[Google Scholar]
|
| 14. |
Alpsoy E, Akman-Karakas A, Uzun S. Geographic variations in epidemiology of two autoimmune bullous diseases: Pemphigus and bullous pemphigoid. Arch Dermatol Res 2015;307:291-8.
[Google Scholar]
|
| 15. |
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5.
[Google Scholar]
|
| 16. |
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
[Google Scholar]
|
| 17. |
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
[Google Scholar]
|
| 18. |
Langan SM, Hubbard R, Fleming K, West J. A population-based study of acute medical conditions associated with bullous pemphigoid. Br J Dermatol 2009;161:1149-52.
[Google Scholar]
|
| 19. |
Ramagopalan SV, Wotton CJ, Handel AE, Yeates D, Goldacre MJ. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: Record-linkage study. BMC Med 2011;9:1.
[Google Scholar]
|
| 20. |
Johannesdottir SA, Schmidt M, Horváth-Puhó E, Sørensen HT. Autoimmune skin and connective tissue diseases and risk of venous thromboembolism: A population-based case-control study. J Thromb Haemost 2012;10:815-21.
[Google Scholar]
|
| 21. |
Cugno M, Marzano AV, Bucciarelli P, Balice Y, Cianchini G, Quaglino P, et al. Increased risk of venous thromboembolism in patients with bullous pemphigoid. The INVENTEP (INcidence of VENous ThromboEmbolism in bullous Pemphigoid) study. Thromb Haemost 2016;115:193-9.
[Google Scholar]
|
| 22. |
Zöller B, Li X, Sundquist J, Sundquist K. Risk of pulmonary embolism in patients with autoimmune disorders: A nationwide follow-up study from Sweden. Lancet 2012;379:244-9.
[Google Scholar]
|
| 23. |
Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie 2010;30:5-6, 8-9.
[Google Scholar]
|
| 24. |
Merkel PA, Lo GH, Holbrook JT, Tibbs AK, Allen NB, Davis JC Jr., et al. Brief communication: High incidence of venous thrombotic events among patients with Wegener granulomatosis: The Wegener's Clinical Occurrence of Thrombosis (WeCLOT) Study. Ann Intern Med 2005;142:620-6.
[Google Scholar]
|
| 25. |
Allenbach Y, Seror R, Pagnoux C, Teixeira L, Guilpain P, Guillevin L; French Vasculitis Study Group. High frequency of venous thromboembolic events in Churg-Strauss syndrome, Wegener's granulomatosis and microscopic polyangiitis but not polyarteritis nodosa: A systematic retrospective study on 1130 patients. Ann Rheum Dis 2009;68:564-7.
[Google Scholar]
|
| 26. |
Stassen PM, Derks RP, Kallenberg CG, Stegeman CA. Venous thromboembolism in ANCA-associated vasculitis – Incidence and risk factors. Rheumatology (Oxford) 2008;47:530-4.
[Google Scholar]
|
| 27. |
Weidner S, Hafezi-Rachti S, Rupprecht HD. Thromboembolic events as a complication of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2006;55:146-9.
[Google Scholar]
|
| 28. |
Johannesdottir SA, Horváth-Puhó E, Dekkers OM, Cannegieter SC, Jørgensen JO, Ehrenstein V, et al. Use of glucocorticoids and risk of venous thromboembolism: A nationwide population-based case-control study. JAMA Intern Med 2013;173:743-52.
[Google Scholar]
|
Fulltext Views
4,959
PDF downloads
3,528





