Translate this page into:
Role of polymerase chain reaction in the diagnosis of Trichomonas vaginalis infection in human immunodeficiency virus-infected individuals from India (South)
2 Department of Dermatology, Christian Medical College, Vellore, Tamil Nadu, India
3 Department of Internal Medicine, Christian Medical College, Vellore, Tamil Nadu, India
4 Department of Community Medicine, Christian Medical College, Vellore, Tamil Nadu, India
5 Department of Clinical Virology, Christian Medical College, Vellore, Tamil Nadu, India
Correspondence Address:
Rajesh Kannangai
Department of Clinical Virology, Christian Medical College, Vellore 632 004, Tamil Nadu
India
| How to cite this article: Paul H, Peter D, Pulimood SA, Abraham OC, Mathai E, Prasad JH, Kannangai R. Role of polymerase chain reaction in the diagnosis of Trichomonas vaginalis infection in human immunodeficiency virus-infected individuals from India (South). Indian J Dermatol Venereol Leprol 2012;78:323-327 |
Abstract
Background: Trichomonas vaginalis is a protozoan parasite and an etiological agent for trichomoniasis, a sexually transmitted infection (STI). Fifty to eighty percentage of women with trichomoniasis are asymptomatic and in the absence of treatment the infection persists longer. Aim: To evaluate the role of polymerase chain reaction (PCR) in the diagnosis of trichomoniasis and also to look at the frequency of infection among human immunodeficiency virus (HIV) infected women. Methods: A non-nested PCR was standardized to detect 102 bp size amplified product of the adhesin gene of T. vaginalis. The real time performance of this assay was performed with vaginal swab samples from 198 HIV-seropositive women who attended the infectious disease clinic and compared with wet mount and culture in Diamond's modified media. Results: Among the prospectively studied 198 HIV-infected women, 1 (0.51%) was positive by wet mount, 6 (3.03%) were positive by culture and 10 (5.02%) were positive by the PCR. There was a significant observed agreement between the PCR and culture (k=0.74, Z=10.7, P<0.0000). Conclusion: Our study showed that the PCR assay for the amplification of adhesion gene is a highly sensitive method to screen the high risk group individuals like HIV-positive women for Trichomonas vaginalis compared to the culture. Testing algorithm should be, wet mount and if negative, test by PCR as it is rapid compared to culture which takes 7 days.Introduction
Trichomoniasis is a common sexually transmitted disease caused by Trichomonas vaginalis has a global incidence of 170 million to 190 million cases annually. [1] South and South East Asia accounts for about 76.5 million new cases reported annually. Symptomatic trichomoniasis is more common in women than in men. It has been estimated that 50% of Trichomonas vaginalis infection among women are asymptomatic. [2],[3],[4] There are several epidemiological studies done in India which showed that trichomoniasis is a major problem. Hospital-based studies done in India showed that the prevalence of Trichomonas vaginalis is as high as 61.7% in women attending STD clinic and as low as 6.7% among women with reproductive tract infections. [5],[6] The most commonly used methods for the diagnosis of trichomoniasis infection are wet mount preparation and culture. [7],[8] The drawbacks of culture are its cost and long duration of incubation required for the growth of the organism. [8] Polymerase chain reaction (PCR) seems to be more practical in diagnosing T. vaginalis infection. The sensitivities of various primers designed, ranges from 85 to 100%. [8] There is insufficient information from India on molecular diagnostic methods and the prevalence of T. vaginalis among the human immunodeficiency virus (HIV) infected individuals. Hence, this study is being undertaken to evaluate the role of PCR in the diagnosis of trichomoniasis among HIV-infected women and also to look at the frequency of infection.
Methods
The study protocol was approved by the institutional review board. A prospective study to detect the frequency of trichomoniasis among HIV-seropositive women was carried out. Vaginal swabs were collected from women attending infectious disease (ID) clinic of a tertiary care hospital and also from a community hospital attached to the same institution. The sampling used was a convenient sampling method. The study period was from the month of June 2006 to February 2007 and later again from November 2007 to July 2008. A total of 204 HIV-seropositive women who attended the two infectious disease clinics for the first time were recruited into the study.
Vaginal discharge was collected by the physicians using three cotton swabs from the posterior vaginal fornix under vision. One swab was used to prepare wet mount preparation to check for the motility of the parasite. Wet mount was checked in the laboratory attached with the ID clinic using a microscope at 10X and 40X objective, respectively, by trained and experienced technologists within 5-8 minutes of sample collection. The second swab was inoculated into the modified Diamond′s media for culture as reported earlier. [3],[9] The inoculated culture broth was incubated at 37°C, and was checked for 10 days, by making wet preparation from broth for characteristic quivering motility of the trophozoites of T. vaginalis every day.
The third swab was inoculated into 2 ml of sterile normal saline in a tube which was vortexed and squeezed well so that all the material collected in the swab was transferred to the saline. This saline was then aliquot into 2 ml screw capped tubes and stored at −20°C for PCR. PCR was then performed in batches with statuses of the culture blinded to the performer of the test. Just prior to the PCR, a stored aliquot was taken, centrifuged in a micro centrifuge at 1200 g for 1 min. The supernatant was discarded and the pellet was reconstituted in about 50 μl of the remaining solution in the tube. DNA was extracted using CHELEX method as previously reported. [10] The extracted DNA was stored at −70°C in multiple aliquots. The primer set used were the TVA5/TVA6 and which amplifies a 102 bp genomic sequence of adhesine gene. [10]
The HotStar Taq Master Mix (QIAGEN, Hielden, Germany) was set up to a final reaction mixture volume of 25 μl by adding sterile distilled water. For each reaction 5 μl of DNA template was added. A touchdown method for thermal cycling was carried out using AB Applied Bio systems thermal cycler (Gene Amp PCR Systems, USA). The product of the PCR was checked for amplification by eletrophoresing in 2% agarose (Sigma Aldrich, Steinhine, USA) gel incorporated with 10 μg/ml (0.01 mM) (10) of ethidium bromide.
Statistical analysis
The accuracy indices of the PCR and the wet preparation in comparison with the broth culture in Diamond′s media were calculated by using the software Epi info. Ver 6.04. The difference in the proportion of the positive samples in asymptomatic and symptomatic HIV-infected women were analyzed by Chi-square test using the software Epi info. Ver 6.04. The Kappa coefficient analysis used to compare the agreement between the performance of culture and the non-nested PCR was carried out by the software Epi info. Ver 6.04.
Results
Among the 204 women recruited for the prospective study, 2 individuals recruited from outside centre were found to be HIV negative on retesting at our hospital. From 2 individuals, swabs which were to be collected for the PCR assay were not available. In another two there was over spillage of samples from the tubes collected for culture and hence discarded. Samples from these 6 individuals were excluded from the study for the analysis purpose. Out of the remaining 198 women, 28 (14.1%) were recruited from the ID clinic of the community hospital while 170 (85.8%) were taken from ID clinic of the main tertiary care hospital. The mean age of the study participants were 33.28 (SD=7.28 yrs, Range 21-52 yrs). Fifty percent of the women were in the age group of 30-49 years of age. Fifty-five (28%) women among the study participants were symptomatic. Twenty-seven among these 55 women presented with vaginal discharge while the remaining 28 were found to have discharge during per vaginal examination.
Among the 198 samples tested by wet mount, only 1 (0.5%) was found to be positive for T. vaginalis trophozoite. Six (3%) of the 198 samples showed positivity in culture. Four of these samples showed positivity in the broth culture by 24 hours. The other two showed positivity within 48 hours.
All the 6 culture positive samples were found to be positive by the non-nested PCR assay. In addition, 4 more samples (total 10 samples, 5%) were found positive by PCR. One of the culture positive samples was negative by the PCR when first tested. However, PCR was repeated with 1/10 and 1/100 diluted DNA input, and the 1/100 diluted sample showed a positive result. A representative gel picture showing the T. vaginalis PCR product with expected band size of 102 bp is shown in [Figure - 1].
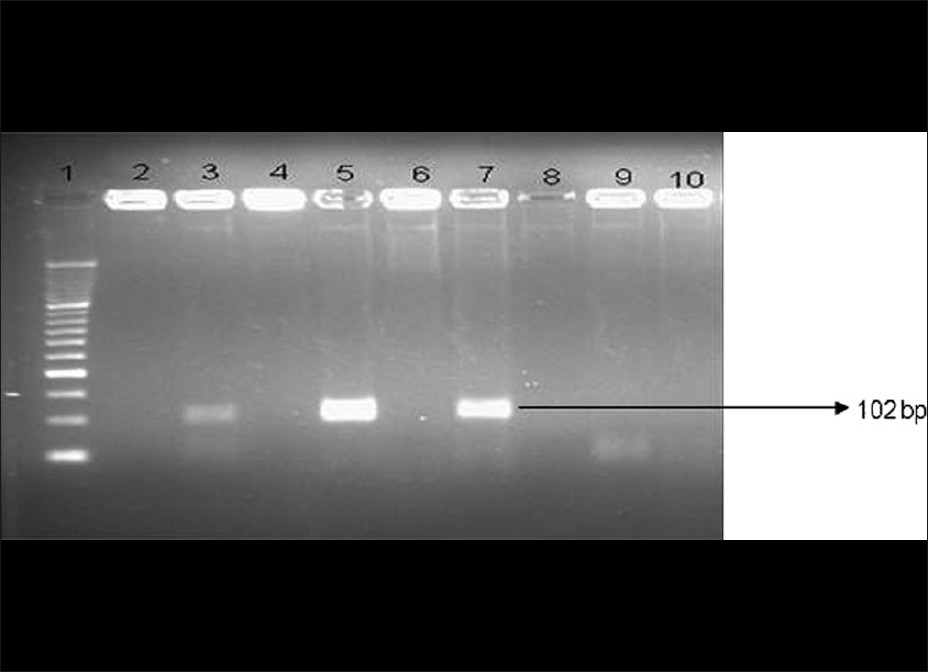 |
| Figure 1: The representative gel picture showing the Trichomonas vaginalis-specific bands polymerase chain reaction assay done on culture - positive samples |
The performance of the culture and the non-nested PCR assay was compared using the Kappa coefficient analysis. There was a significant observed agreement of 98% (kappa coefficient of 0.74) between the PCR and culture (Z value 10.79, P value <0.001).
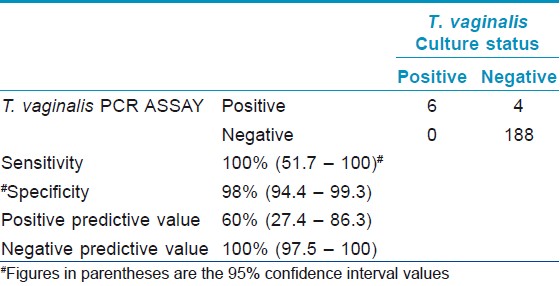
Proportion of samples positive (5.5%) by any one tests in symptomatic (n=55) individuals were higher (4.9%) than asymptomatic individuals (n=143). However, this difference was not significant (P=<0.05). Considering culture as gold standard, the sensitivity of the wet mount was only 16.6% with 100% specificity. The non-nested PCR assay had a sensitivity of 100% and a specificity of 98% when compared with the broth culture. The accuracy indices of the PCR assay in comparison to the culture is shown in [Table - 1].
Discussion
There are number of tests that can be used for the diagnosis of trichomoniasis. These include vaginal wet preparation, vaginal swab culture and vaginal swab PCR. Apart from vaginal swabs, urine can also be used as a specimen for the diagnosis by any one of the mentioned tests but had a lower sensitivity. [8],[11],[12] The reported sensitivity of the wet preparation for the T. vaginalis was about 56%. [8],[13] The sensitivity of the wet preparation was quite poor in our study as well. One of the disadvantages with this low sensitivity of detection of Trichomonas vaginalis among HIV infected women by the routine wet preparation is that many infected women may not receive appropriate timely treatment. This will lead to complications in infected individuals, transmission to sexual partners, and can even increase the transmission of HIV. The increased transmission may be because of the disruption of urogenital epithelial cells caused by T. vaginalis that facilitates the passage of HIV and also due to the activation of local immune cells leading to increased HIV replication. [14] These findings suggest the need for a more efficient and sensitive method of screening trichomoniasis especially in HIV-infected individuals.
There are several studies which looked at the usability of polymerase chain reaction (PCR) for the detection of T. vaginalis. In the study reported here, we looked at the usefulness of PCR in the detection of trichomoniasis in a cohort of HIV-infected women. The primer set used in this study amplifies a 102 bp genomic sequence termed as A6p. This area is a much conserved segment of the adhesin gene and hence the PCR using primer set of TVA5/TVA6 was proved to be highly selective for a wide range of T. vaginalis isolates. [10] During the standardization, we tested 40 T. vaginalis culture negative and 30 culture positive samples and the PCR assay showed a sensitivity and specificity of 96.7% and 97.5%, respectively. In our prospective study a total of 10 samples were found to be positive by the PCR including the six culture-positive samples. However, one of the culture-positive sample became positive only after repeating the PCR with a 1/100 diluted DNA input. This particular sample was also positive by the direct wet mount preparation. This indicated a larger number of parasites in the original sample. Hence, the lack of amplification in the undiluted and 1/10 diluted sample may be due to template DNA excess. The template excess may cause chelation of Mgcl 2 added to suboptimal level for the results for the PCR reactions. Another reason may be presence of some PCR inhibitor that should have gone away with dilution of the sample. Compared to the culture the PCR assay used in our study was found to have significant observed agreement (Z=10.49, P<0.001) with a Kappa coefficient 0.74. Moreover the assay was able to detect 4 additional samples than culture. Although the combination of culture and wet mount examination remains the standard approach for detecting T. vaginalis in patient samples, there is no gold standard for confirmation. Wet preparation is the test widely available in most of the STD clinics it is cheaper (Cost <100) but its sensitivity was poor. Broth culture technique using Diamond′s medium is considered as the ′gold standard′ for the diagnosis of trichomoniasis. The minimum inoculum size required for a positive result is about 102 organisms/ml and the growth of the organism is easy to interpret. [15] However, it requires special media, faster transportation of the sample to the laboratory, costlier (approximate cost 700) and the results are available only within 2-7 days. [9],[15] Wendel et al., has shown that diagnosis of trichomoniasis by means of wet preparation and other factors like treatment of known recent contacts and pelvic inflammatory diseases resulted in the treatment of only 69% of T. vaginalis infected women while the use of T. vaginalis PCR would have allowed treatment of a significantly high (84%) number of infected women. [16] PCR (approximate cost 1000) would improve identification and treatment of even asymptomatic trichomoniasis and this will able to reduce vaginal HIV RNA levels in HIV-infected women and in turn help to prevent or reduce the further transmission of HIV.
The prevalence of T. vaginalis among the HIV-infected individuals also varied from region to region. In a case controlled study reported from Nigeria, among the HIV infected and uninfected mothers 18.6% of HIV-infected women and 10.2% of HIV-uninfected women were shown to have T. vaginalis infection. [17] The frequency of T. vaginalis in HIV-seropositive Nigerian women was found to be 24.4% while from developed countries the prevalence among HIV-infected individuals varied from 11 to 36%. [18],[19],[20],[21] Our study has shown the frequency of trichomoniasis in HIV-infected women as 5.05% with PCR or any one assay positive. We expected a higher frequency of T. vaginalis among the HIV-infected women as reported from Africa and developed countries. [17],[18],[19],[20],[21] However, the frequency observed was almost similar to the frequency (6%) seen among women in antenatal clinic but lower (13%) than that reported from a rural community and STI clinic attendees of neighboring district of Tamil Nadu. [3],[22],[23] The possible reason for this low prevalence could be attributed to the selection of patients for the study. Since the study setups were in tertiary care center and relatively expensive, only patients who can afford the management may come to the hospital. A good number of patients among the 198 women in our study were either referred from other hospitals or from other clinics in this hospital to the infectious disease clinic. Four (67%) out of 6 women tested positive by culture and seven (70%) out of 10 women tested positive by PCR assay were asymptomatic. It is possible that women who came to our hospital who had symptoms suggesting T. vaginalis infection would have been treated by the general physicians elsewhere with metronidazole and were negative when tested here. The study done in high risk groups with other sexually transmitted infections would have possibly shown a different picture.
In conclusion, our study clearly showed that the PCR assay using the TAV5/TAV6 primer set is a highly sensitive method for the detection of T. vaginalis compared to the culture. It is important to screen the high risk group individuals like HIV-positive women for T. vaginalis routinely with highly sensitive techniques like PCR as most of the individuals are asymptomatic and may not receive appropriate treatment. Lack of timely management of the infection may lead to complications in infected individuals and also can increase the transmission of HIV to partners and newborns. The testing algorithm should be wet mount and if negative, test by PCR as it is more sensitive and rapid compared to culture which takes 7 days.
Acknowledgement
We would like to acknowledge the fluid research fund of Christian Medical College, Vellore for the partial funding of this study.
| 1. |
Vander Pol B. Trichomonas vaginalis infection: The most prevalent non-viral sexually transmitted infection receives the least public health attention. Clin Infect Dis 2007;44:23-5.
[Google Scholar]
|
| 2. |
Johnston VJ, Mabey DC. Global epidemiology and control of Trichomonas vaginalis. Curr Opin Infect Dis 2008;21:56-64.
[Google Scholar]
|
| 3. |
Mathai E, Muthaiah A, Mathai M, Jasper P. Prevalence and effects of trichomoniasis in pregnancy. Natl Med J India 1998;11:151.
[Google Scholar]
|
| 4. |
Desai VK. Prevalence of sexually transmitted infections and performance of STI syndromes against an etiological diagnosis in female sex workers of red light area in Surat India. Sex Transm Infect 2003;79:111-5.
[Google Scholar]
|
| 5. |
Agrawal BM, Agarwal S, Singh PK, Rizvi G. Trichomonas vaginalis - An indicator for other sexually transmitted infecting agents. Indian J Dermatol Venereol Leprol 2000;66:241-3.
[Google Scholar]
|
| 6. |
Sood S, Mohanty S, Kapil A, Tolosa J, Mittal S. InPouch TV culture for detection of Trichomonas vaginalis. Indian J Med Res 2007;125:567-71.
[Google Scholar]
|
| 7. |
Elhoussieny TR. Trichomonas vaginalis. Am J Obstet Gynecol 2004;1:107-9.
[Google Scholar]
|
| 8. |
Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev 2004;17:794-803.
[Google Scholar]
|
| 9. |
Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology. Washington: American Society for Microbiology; 1995.
[Google Scholar]
|
| 10. |
Madico G, Quinn TC, Rompalo A, McKee KT, Gaydos CA. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J Clin Microbiol 1998;36:3205-10.
[Google Scholar]
|
| 11. |
Lawing LF. Detection of trichomoniasis in vaginal and urine specimens from women by culture and PCR. J Clin Microbiol 2000;38:3585-8.
[Google Scholar]
|
| 12. |
Mohamed OA, Cohen CR, Kungu D, Kuyoh MA, Onyango JA, Bwayo JJ, et al. Urine proves a poor specimen for culture of Trichomonas vaginalis in women. Sex Transm Infect 2001;77:78-9.
[Google Scholar]
|
| 13. |
Draper D, Parker R, Patterson E, Jones W, Beutz M. Detection of Trichomonas vaginalis in pregnant women with the InPouch TV culture system. J Clin Microbiol 1993;31:1016-8.
[Google Scholar]
|
| 14. |
Guenthner PC, Secor WE, Dezzutti CS. Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: Implications for the sexual transmission of HIV-1. Infect Immunol 2005;73:4155-60.
[Google Scholar]
|
| 15. |
Stary A, Kuchinka-Koch A, Teodorowicz L. Detection of Trichomonas vaginalis on modified Columbia agar in the routine laboratory. J Clin Microbiol 2002;40:3277-80.
[Google Scholar]
|
| 16. |
Wendel KA, Erbelding EJ, Gaydos CA, Rompalo AM. Trichomonas vaginalis polymerase chain reaction compared with standard diagnostic and therapeutic protocols for detection and treatment of vaginal trichomoniasis. Clin Infect Dis 2002;35:576-80.
[Google Scholar]
|
| 17. |
Sutton MY, Sternberg M, Nsuami M, Behets F, Nelson AM, St Louis ME. Trichomoniasis in pregnant human immunodeficiency virus-infected and human immunodeficiency virus-uninfected Congolese women: Prevalence, risk factors, and association with low birth weight. Am J Obstet Gynecol 1999;181:656-62.
[Google Scholar]
|
| 18. |
Uneke CJ, Alo MN, Ogbu O, Ugwuoru DC. Trichomonas vaginalis infection in human immunodefiency virus seropositive Nigerian women: The public health significance. Online Journal of Health and Allied Sciences. Available from: http://www.ojhas.org/issue22/2007-2-32007. [Last Accessed on 2011 May 17].
[Google Scholar]
|
| 19. |
Bersoff-Matcha SJ, Horgan MM, Fraser VJ, Mundy LM, Stoner BP. Sexually transmitted disease acquisition among women infected with human immunodeficiency virus type 1. J Infect Dis 1998;178:1174-7.
[Google Scholar]
|
| 20. |
Cu-Uvin S, Hogan JW, Warren D. Prevalence of lower genital tract infections among human immunodeficiency virus (HIV)-seropositive and high-risk HIV- seronegative women. HIV Epidemiology Research Study Group. Clin Infect Dis 1999;29:1145-50.
[Google Scholar]
|
| 21. |
Sorvillo F, Kovacs A, Kerndt P. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: Implications for HIV prevention. Am J Trop Med Hyg 1998;58:495-500.
[Google Scholar]
|
| 22. |
Prasad JH, Abraham S, Kurz KM, George V, Lalitha MK. Reproductive tract infections among young married women in Tamil Nadu, India. Int Fam Plan Perspect 2005;31:73-82.
[Google Scholar]
|
| 23. |
Krishnamurthy VR, Ramachandran V. STD trends in Chengalpattu. Indian J Dermatol Venereol Leprol 1996;62:3-12.
[Google Scholar]
|
Fulltext Views
2,374
PDF downloads
2,212





