Translate this page into:
Safety and efficacy of autologous noncultured dermal cell suspension transplantation in the treatment of localized facial volume loss: A pilot study
2 Department of Radiology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Somesh Gupta
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi
India
| How to cite this article: Sahoo AK, Yadav S, Sharma VK, Parihar AS, Vyas S, Gupta S. Safety and efficacy of autologous noncultured dermal cell suspension transplantation in the treatment of localized facial volume loss: A pilot study. Indian J Dermatol Venereol Leprol 2019;85:44-50 |
Abstract
Background: Available options for correction of facial volume loss, such as synthetic fillers, autologous fat and cultured fibroblasts, have limitations viz. temporary effect and high cost.
Aim: To assess the use of a novel technique, autologous non-cultured dermal cell suspension transplantation, for correction of localized facial volume loss due to inflammatory pathologies.
Methods: It was a pilot study conducted in the Dermatology Outpatient Department, All India Institute of Medical Sciences (AIIMS), New Delhi, India. Autologous non-cultured dermal cell suspension was transplanted in a total of 10 patients, out of which 5 had predominantly dermal loss and the rest had predominantly lipoatrophy. The donor tissue from the gluteal region was digested into a single cell suspension using collagenase-1 and injected into the recipient area. The outcome was assessed subjectively by patients and investigators and objectively using ultrasonography. Cell count, viability testing and measurement of mesenchymal stem cells were also done.
Results: On assessment of patients, the median improvement in the predominantly dermal atrophy group at 3 and 6 months was 70% (range: 10–90%) and 80% (range: 0–90%), respectively, and in the predominantly lipoatrophy group, 0% (range: 0–40) and 0% (range: 0–50), respectively. Mean thickness of dermis + subcutis at the baseline was 1.835 mm (range: 0.89–6.04 mm), which increased to 2.912 mm (range: 0.88–7.07 mm, P = 0.03) at 6 months.
Limitations: Our pilot study has some limitations such as small sample size and heterogeneity of the recruited patients.
Conclusions: Autologous non-cultured dermal cell suspension transplantation appears to be safe and effective in localized facial dermal defects because of inflammatory pathologies, but not effective in deeper defects.
Introduction
The evolution of medical care and improvements in personal health have allowed people to live longer, healthier and more productive lives. As a consequence, society now places great value on maintaining a youthful appearance. In addition to aging, many inflammatory conditions such as acne vulgaris, morphea and lupus profundus can leave behind unacceptable volume loss on face. Available options to treat these conditions are synthetic dermal fillers, autologous fat transplantation and the recently introduced technique of autologous cultured fibroblast transplantation.[1],[2] There are certain problems associated with synthetic dermal fillers, they being foreign substances to the human body. These are: foreign body reaction, granuloma formation, migration to other areas and infection and abscesses.[3] Another significant drawback of these fillers is that the effect is temporary as they are degraded by host enzymes such as collagenase and hyaluronidase, requiring repeated injections with their associated cost.[4] Filling effect with synthetic fillers is due to their volume and not because of regeneration of lost or damaged tissue. An ideal filler would be one which restored the volume by regeneration, replacing like with like, was autologous, long-lasting, not technology- intensive and had minimal risk of complications. To meet these criteria, the emphasis should be on cell-based therapy. Autologous fat transfer is widely practiced for restoration of facial volume loss. Though this technique has evolved over time, the results are still inconsistent, with variable longevity, particularly when volume loss is predominantly due to loss of dermal tissue.[5]
In this pilot study, we have assessed the efficacy and safety of a novel technique, autologous noncultured dermal cell suspension transplantation for correction of localized facial volume loss due to various diseases.
Methods
Ten patients having localized visible volume loss (size <5 cm) on the face as a result of a disease or inflammation were recruited for the study after obtaining institutional ethical clearance. Only patients in whom the underlying pathology was quiescent, as evidenced by clinical remission for at least 6 months, were included in the study. Patients with keloidal tendency, bleeding diathesis, active infection at the treatment site, those on any immunosuppressive therapy and pregnant and lactating women were excluded from the study. Excision biopsy of size 5 × 2 cm from an unexposed area (such as buttock or thigh) was obtained after removing the epidermis by a motorized dermabrader. Fat globules adhered to dermal tissue were removed with scissors, and the intact dermis was transported to the laboratory using a transport medium, Dulbecco's modified Eagle's medium (Sigma-Aldrich, St Louis, MO, USA), pH 7.2, supplemented with penicillin, streptomycin and amphotericin-B (Gibco BRL, Gaithersburg, MD, USA). All laboratory procedures were done under strict aseptic conditions in a biosafety cabinet. After washing with phosphate buffer saline, the dermal tissue was minced with the help of a surgical blade or scissors. Collagenase solution was prepared by adding lyophilized form of collagenase (concentration: 280 U/mg) to phosphate buffer saline to form a final concentration of 3 mg/ml. After weighing the minced dermal tissue, 5 ml of collagenase solution was added per gram of dermal tissue and it was incubated with collagenase type 1 (280 units/mg, Sigma-Aldrich, St Louis, MO, USA; final concentration 3 mg/ml) in CO2 incubator at 37°C for a minimum of 4 h. After the incubation, the dermal tissue was washed again with phosphate buffer saline and filtering of cell suspension was done using 70 μm cell strainer (BD Falcon, MA 01730, USA). After that, spinning of cell suspension was done at 3000 rpm at 4°C for 10 min that led to the formation of a cell pellet at the bottom of the test tube. Supernatant was discarded and the pellet was re-suspended in small amount of Dulbecco's modified Eagle's medium and was sent to the operating room for transplantation [Figure - 1], [Video 1]. Small amount of autologous noncultured dermal cell suspension was used for cells counting using Neubauer's chamber hemocytometer, and cell viability testing by staining with trypan blue and a small portion was preserved for quantification of dermal mesenchymal stem cells. The recipient area was surgically cleansed and anesthetized using topical anesthetic cream (eutectic mixture of local anesthetics). Then autologous noncultured dermal cell suspension was injected into the dermis very superficially using 18G needle by multiple linear thread technique. [Video 2] On an average, 4–5 ml of dermal cell suspension was injected per se ssion. After injection of dermal cell suspension, the entry point of the needle on recipient area was sealed with steri strip®. Patients were given up to three injections at monthly intervals. Patients were asked to come for evaluation after 7 days of every injection; then at 1, 2, 3 and 6 months from the first injection. Photographs were taken at all the visits. Improvement was assessed subjectively by the patients in terms of percentage improvement from the baseline. In addition, blinded and randomized evaluation of photographs taken at baseline, 3 and 6 months was done by two independent assessors, both experienced dermatologists. Ultrasonographic measurement of combined thickness of the dermis and subcutaneous tissue at the site of the volume loss as well as in the surrounding normal tissue was done using high-frequency ultrasonography (Philips I 2, 12 MHz linear probe) at baseline and at 6 months. Quantitative assessment of mesenchymal stem cells was done by flow cytometry. Mesenchymal stem cells were defined as cells positively expressing CD 73, CD 90, CD 105, and negative for CD34 and CD45.
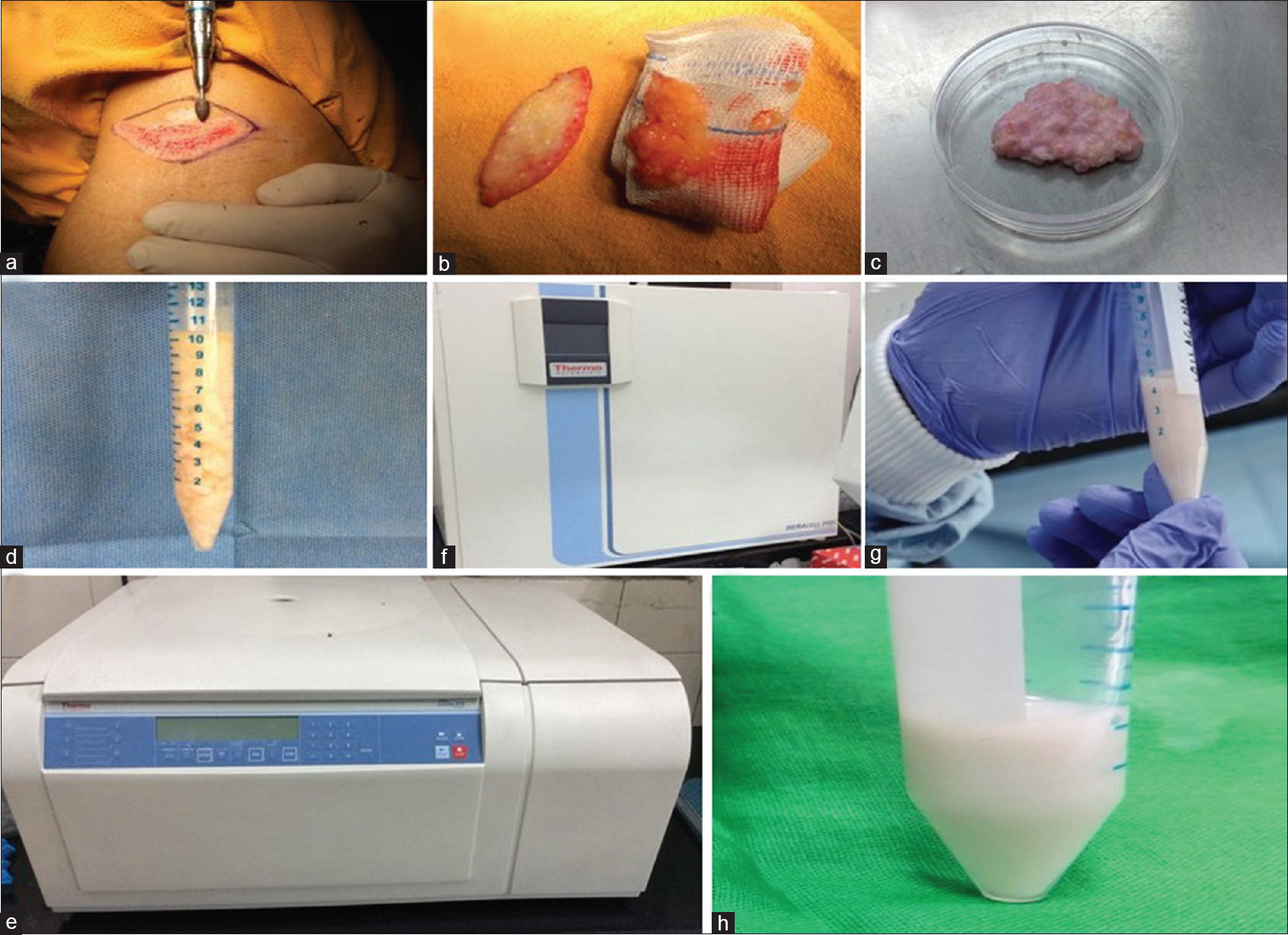 |
| Figure 1: (a) Steps of method of preparation of autologous noncultured dermal cell suspension. Removal of epidermis by motorized dermabrader (b) Excision elliptical biopsy from covered site with complete removal of fat from the dermal tissue (c) Minced dermal tissue (d) Addition of collagenase to minced dermal tissue (e) Incubation in CO2 incubator for 4 h (f) Enzymatically digested dermal tissue (g) Centrifugation at 3000 rpm for 10 min (h) Final re-suspended dermal cell suspension |
Results
All the patients (6 males, 4 females) included in the study completed 6 months of follow-up. Age of the patients ranged from 15 to 24 years (mean: 20.8 ± 3.35 years). The demographic profile of patients, indication for treatment and the number of injections received are shown in [Table - 1]. Volume loss was classified into two broad categories, namely predominantly dermal and predominantly lipoatrophy [Table - 1]. Out of 10 patients, 4 received three injections, 4 received two injections (not willing to undergo further due to adequate improvement) and the remaining 2 received a single injection (not willing for more injections due to no improvement). Mean weight of the dermal tissue taken was 1.45 ± 0.25 g (range: 1.1–1.8 g). Mean cumulative volume of dermal suspension injected was 10.3 ± 3.86 ml (range: 4–15 ml). Mean cell count was 1.49 ± 0.22 million cells/ml (range: 1.3 − 1.9 × 106 per ml). The mean percentage cell viability was 88.7 ± 2.1 (range: 84–91). However, no correlation was found between improvement in volume loss with the weight and volume of the dermal tissue, cell count and viability. For measurement of mesenchymal stem cell population in dermal cell suspension, samples of all patients were analyzed by flow cytometer. Each sample was subjected to analysis using specific antibodies with negative expression of CD34 and CD45, and positive co-expression of CD73, CD90 and CD105. The mean percentage of dermal mesenchymal stem cell population was 0.7 ± 0.52 (range: 0–1.6).

In patients' assessment, the median improvement at 3 months was 40% (range: 0–90%) and at 6 months was 50% (range: 0–90%). Out of 10 patients, 5 were classified as predominantly dermal atrophy and remaining 5 as predominantly lipoatrophy based on clinical and ultrasonographic assessment. The median improvement in the predominantly dermal atrophy group at 3 and 6 months were 70% (range: 10–90%) and 80% (range: 0–90%), respectively and in the predominantly lipoatrophy group, 0% (range: 0–40) and 0% (range: 0–50) at 3 and 6 months, respectively. The difference in the improvement between these two groups was statistically significant, both at 3 and 6 months (P = 0.02, 0.05, respectively) [Figure - 2]a and [Figure - 2]b.
 |
| Figure 2: (a) Patients' assessment of percentage improvement at 3 months in predominantly dermal atrophy group and predominantly lipoatrophy group (b) Patients' assessment of improvement at 6 months (c) Comparison of the combined thickness of dermis and subcutis before and 6 months (d) Comparison of the thickness of dermis and subcutis combined before and 6 months after dermal cell suspension injection in two groups, namely predominantly dermal atrophy group and predominantly lipoatrophy group fter dermal cell suspension injections, in all patients together |
In the assessment by dermatologists, the median subjective improvement at 3 months was 55% (range: 20–80%) and 50% (range: 10–80%), respectively, and at 6 months, 50% (range 10–80%) and 40% (range 20–90%), respectively. Comparison of the improvement between the two groups in dermatologists' assessment was not significantly different (P value: 0.39, 0.20, respectively).
Mean thickness of dermis and subcutis together at the defect site at the baseline was 1.835 mm (range: 0.89–6.04 mm) and at 6 months 2.912 mm (range: 0.88–7.07 mm) (P = 0.03) [Figure - 2]c. Among the two groups, in the pure dermal atrophy group mean thickness of the dermis and subcutis combined at the baseline was 1.63 mm (range: 1.42–6.04 mm) and at 6 months was 3.14 mm (range: 1.66–7.07 mm, P = 0.06). In the predominantly lipoatrophy group, the respective figures were 1.97 mm (range: 0.89–4.35 mm) and 2.14 mm (range: 0.88–4.85 mm, P = 0.46), respectively [Figure - 2]d.
Spearman correlation coefficient (r) between assessments by dermatologists 1 and 2 with that of patients was 0.85 and 0.98, respectively, and with the ultrasonography assessment was 0.55 and 0.67, respectively. The correlation coefficient between patient assessment and ultrasonography measurement was 0.61.
Immediate complications noted were erythema, edema and mild to moderate pain at the site of injection in all the patients, which persisted for few hours. All patients were prescribed analgesics for a period of 2–3 days and oral antibiotics for a period of 1 week. One patient developed infection at the donor site resulting in wound dehiscence, which was successfully managed with oral antibiotics and local wound care. No long-term complications were noted at both the recipient and donor sites. The donor site healed with a linear surgical scar which became less conspicuous at 6 months follow-up. One patient of lupus panniculitis had reactivation of the disease in the form of development of around 4–5 subcutaneous mildly tender nodules on back and thigh at around 10 months after the first injection of dermal cell suspension.
Discussion
At present, the available options for volume restoration are synthetic fillers, autologous fat transplantation and recently introduced autologous cultured fibroblast therapy. When the defect is primarily in the dermis, its ideal replacement should be with the dermal tissue itself. Fibroblasts constitute the major functional cells in the dermis, which synthesize and deposit collagen. They are the most resilient and easily cultivable cells among all the cells in the skin. Autologous cultured fibroblasts injection is the first and the only autologous cell therapy approved by the United States Food and Drug Administration for esthetic use.[2] The technique of autologous fibroblast transplantation involves taking small post auricular punch biopsy followed by culture expansion of fibroblasts and injection into the dermis of recipient site. It is found to be effective with results persisting for at least 12–48 months and without any serious adverse effects.[6],[7] Histologic analysis in these studies demonstrated that fibroblast injections increased collagen formation, accompanied by a concomitant increase in thickness and density of dermal collagen. This has been tried for the correction of nasolabial fold and superficial age-related wrinkles with significant improvement.[8],[9] However, culture techniques are time-consuming, technically demanding, expensive and require sophisticated, fully equipped, dedicated cell culture laboratory and trained personnel, resulting in high cost of the treatment. The use of various growth factors and additives in the culture medium raises safety concerns. In addition, there is a long interval between harvesting the donor tissue to injection at the recipient site. Along with fibroblasts, the dermis also contains ground substance, which consists of water, electrolytes, protein and muco-polysaccharides namely glycosaminoglycan (consisting of hyaluronic acid, heparin sulfate and small amount of chondroitin sulfate). Glycosaminoglycan constitutes most of the volume of the ground substance of the dermis due to it's water retention capacity.[10] The dermis also represents a larger reservoir for adult stem cells than the epidermis and hair follicle together.[11] Also, it is the second most abundant source of mesenchymal stem cells, next only to the bone marrow. These cells are pluripotent cells which can differentiate into various cell lines including fibroblast and adipocytes.[5]
Our study is a pilot study of a novel cell-based therapy, autologous noncultured dermal cell suspension transplantation, in 10 patients having volume loss due to inflammatory pathologies. Five patients (three cases of postacne scarring causing depression of cheeks, one each of posttraumatic scar and postfuruncle volume loss) had predominantly dermal atrophy and in remaining 5 (3 cases of morphea, 2 cases of lupus panniculitis) had predominantly lipoatrophy. All the patients in the latter group had stable disease for more than 6 months and were off immunosuppressive therapy. Autologous non-cultured dermal cell suspension was prepared and injected on the same day. Patients with predominantly dermal atrophy had median improvement of 70 and 80% at 3 and 6 months, respectively, as per patient assessment which correlated well with the assessment of two dermatologists as well as with objective assessment by ultrasonography. Although the endpoint of the study was 6 months, three patients (a case of post furuncle depressed scar, post traumatic scar and a post acne scar) who came at 1 year post injections showed persistent improvement [Figure - 3], [Figure - 4], [Figure - 5]. On the contrary, those having predominantly lipoatrophy had no significant improvement [Figure - 6]. The ideal filler for correction of volume loss should be one that replaces the lost tissue with identical autologous tissue. It should also be safe, inexpensive and long-lasting. In volume loss primarily due to loss of dermal tissue, the replacement should be done with autologous dermal tissue. As dermis contains pluripotent dermal mesenchymal stem cells which have the potential to differentiate into adipocytes, we presumed that autologous non-cultured dermal cell suspension would help in correction of lipoatrophy as well. However, the number of stem cells was too low in the cell suspension and those patients who had both dermal and subcutaneous tissue loss probably needed layered injections of autologous fat and dermal cell suspension together for lasting improvement. As the objective of our study was to assess the efficacy and safety of autologous non-cultured dermal cell suspension in the correction of facial volume loss, we included only dermis for preparation of the cell suspension. Though the predominant cells in the dermis are fibroblasts, it also contains dermal mesenchymal stem cells that have the potential to differentiate into various cell lines including adipocytes. So, we presumed that dermal cell suspension would help in correction of lipoatrophy as well. However, it helped only in dermal defects and not in deeper subcutaneous defects. Another interesting observation in our study was that those patients who had improvement after initial injections, the effect persisted at 3 months, and at times improved even more at 6 months post injection [Figure - 3]. This suggested that it is truly a regenerative cell-based therapy rather than merely a filling effect. Possibly the cells (fibroblast, dermal mesenchymal stem cells) in the suspension proliferated in their natural milieu leading to neo-collagenesis, and deposition of ground substance resulting in sustained correction of volume defect.
 |
| Figure 3: (a) Postfuruncle volume loss (predominantly dermal atrophy) at baseline (b) Postfuruncle volume loss (predominantly dermal atrophy) at 3 months showing 70% improvement from the baseline (c) Postfuruncle volume loss (predominantly dermal atrophy) at 6 months showing 90% improvement from the baseline |
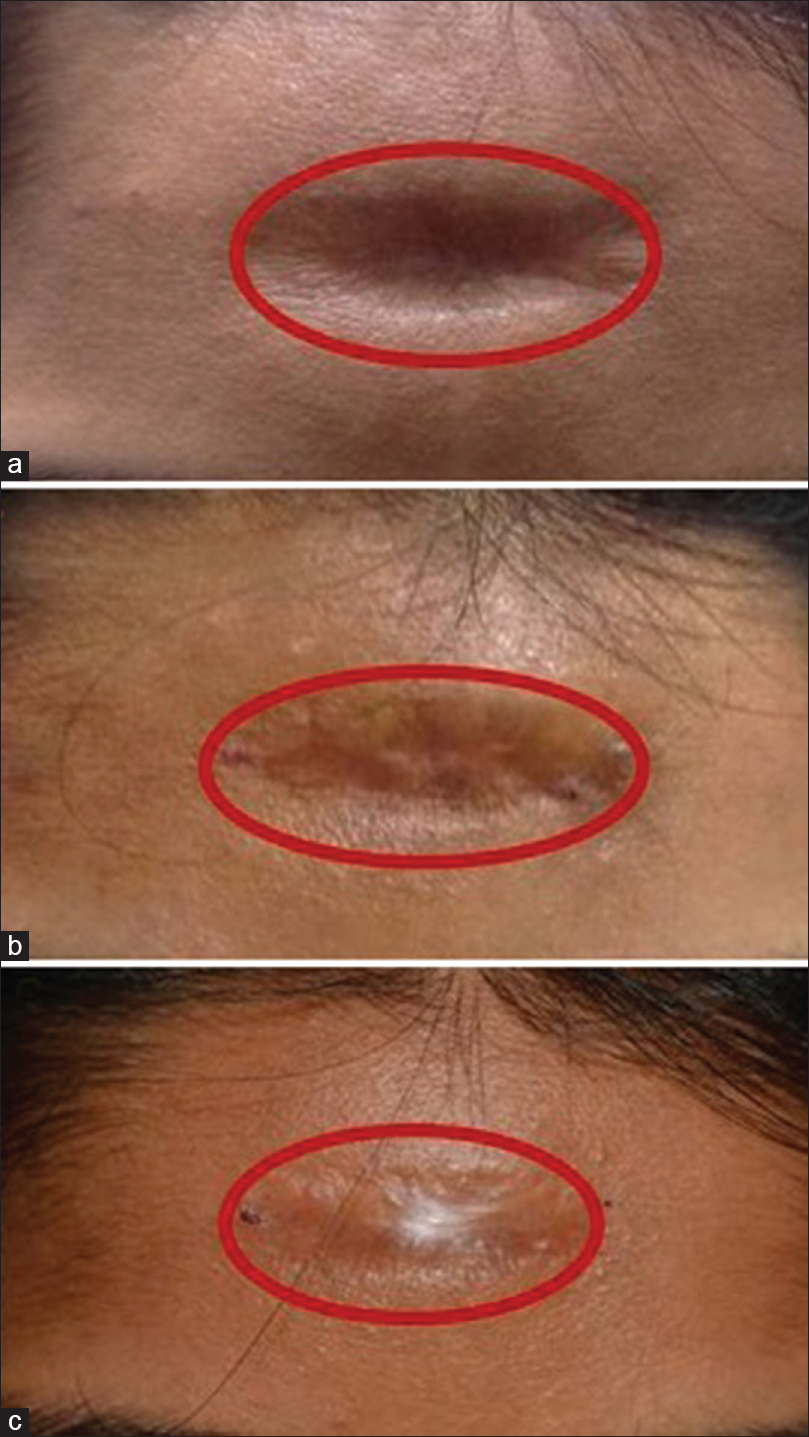 |
| Figure 4: (a) Posttraumatic scar (predominantly dermal atrophy) at baseline (b Posttraumatic scar (predominantly dermal atrophy) at 3 months showing 80% improvement from the baseline) (c) Posttraumatic scar (predominantly dermal atrophy) at 6 months showing 90% improvement from the baseline |
 |
| Figure 5: (a) Postacne scar (predominantly dermal atrophy) at baseline (b) Postacne scar (predominantly dermal atrophy) at 3 months showing 50% improvement from the baseline (c) Postacne scar (predominantly dermal atrophy) at 6 months showing 70% improvement from the baseline |
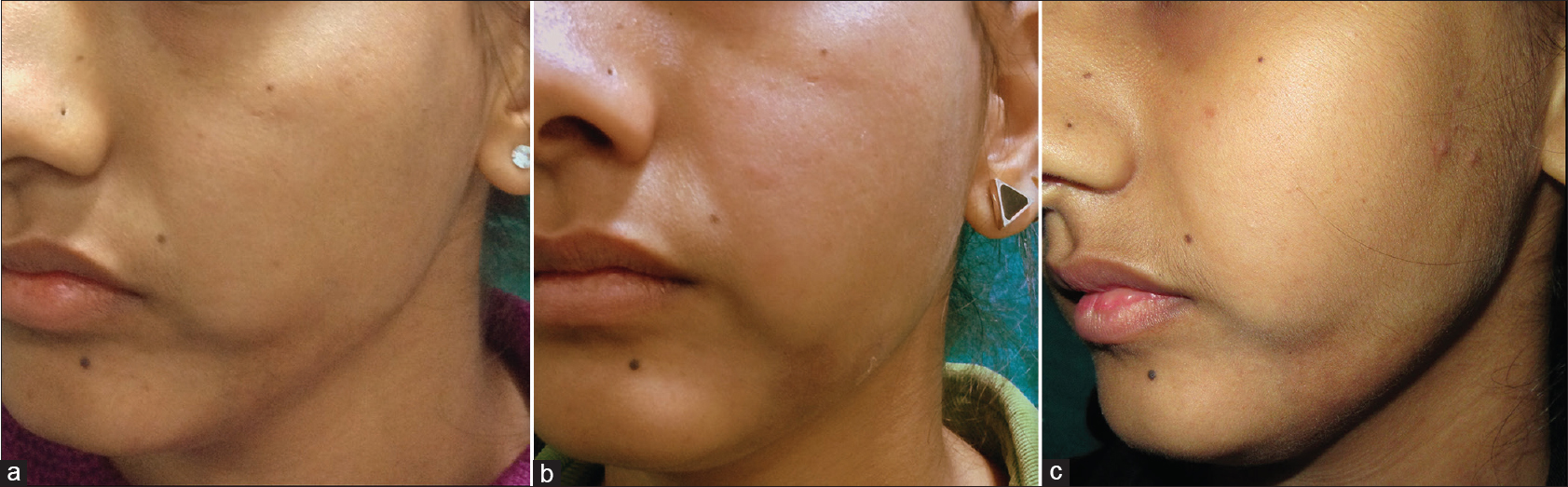 |
| Figure 6:(a) Localized morphea at baseline (b) Localized morphea at 3 months, showing no improvement from baseline (c) Localized morphea at 6 months, showing no improvement from baseline |
In our study, mesenchymal stem cells were defined by positive co-expression of CD73, CD90 and CD105, and negative expression of CD34 and CD45. The mean percentage of mesenchymal stem cells was 0.7 ± 0.52. Currently, there is no consensus on surface markers to define the mesenchymal stem cells. The definition based on the combination of surface markers chosen by us is the most acceptable one in the current literature.[12] The mean cell count was 1.49 ± 0.22 million cells/ml. The cellular component of the dermis includes predominantly fibroblasts along with some other cells such as dermal mesenchymal stem cells, chondrocytes, osteocytes, inflammatory cell infiltrates such as lymphocytes and histiocytes. However, individual identification of cell morphology cannot be done by light microscopy. It needs special staining which has not been done in our pilot study. Our study has some other limitations as well such as small sample size and heterogeneity of the recruited patients. Some patients had predominantly dermal atrophy and some had predominantly lipoatrophy. The injecting protocol couldn't be adhered strictly as many patients refused to undergo further injections, either due to adequate improvement or no improvement after initial injection(s).
Conclusion
Autologous non-cultured dermal cell suspension appears to be effective in localized facial volume loss predominantly due to loss of dermal tissue as a result of inflammatory pathologies. However it is not effective in facial volume loss due to loss of subcutaneous fat and underlying muscles. A larger randomized controlled trial is needed to generate stronger evidence for its use in localized facial volume loss due to loss of dermal tissue as well as in aesthetic indications, such as facial volume loss due to ageing.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Acknowledgement
We are grateful to Dr B. K. Khaitan for providing valuable inputs during our study, Dr Bimal Das and Dr Ravinder Singh for helping us in flow cytometry, Mr Ashish D. Upadhyay for doing statistical analysis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Gupta K, Bhari N, Verma KK, Gupta S. Permanent injectable polyacrylamide hydrogel dermal filler for a large subcutaneous defect secondary to lupus panniculitis. Dermatol Surg 2017;43:152-4.
[Google Scholar]
|
| 2. |
Weiss RA. Autologous cell therapy: Will it replace dermal fillers? Facial Plast Surg Clin North Am 2013;21:299-304.
[Google Scholar]
|
| 3. |
Rongioletti F, Cattarini G, Sottofattori E, Rebora A. Granulomatous reaction after intradermal injections of hyaluronic acid gel. Arch Dermatol 2003;139:815-6.
[Google Scholar]
|
| 4. |
Dover JS, Rubin MG, Bhatia AC. Review of the efficacy, durability, and safety data of two nonanimal stabilized hyaluronic acid fillers from a prospective, randomized, comparative, multicenter study. Dermatol Surg 2009;35 Suppl 1:322-30.
[Google Scholar]
|
| 5. |
Scarborough DA, Schuen W, Bisaccia E. Fat transfer for aging skin: Technique for rhytids. J Dermatol Surg Oncol 1990;16:651-5.
[Google Scholar]
|
| 6. |
Watson D, Keller GS, Lacombe V, Fodor PB, Rawnsley J, Lask GP, et al. Autologous fibroblasts for treatment of facial rhytids and dermal depressions. A pilot study. Arch Facial Plast Surg 1999;1:165-70.
[Google Scholar]
|
| 7. |
Boss WK Jr., Usal H, Chernoff G, Keller GS, Lask GP, Fodor PB, et al. Autologous cultured fibroblasts as cellular therapy in plastic surgery. Clin Plast Surg 2000;27:613-26.
[Google Scholar]
|
| 8. |
Smith S, Busso M, McClaren M, Bass LS. A randomized, bilateral, prospective comparison of calcium hydroxylapatite microspheres versus human-based collagen for the correction of nasolabial folds. Dermatol Surg 2007;33 Suppl 2:S112-21.
[Google Scholar]
|
| 9. |
Eça LP, Pinto DG, de Pinho AM, Mazzetti MP, Odo ME. Autologous fibroblast culture in the repair of aging skin. Dermatol Surg 2012;38:180-4.
[Google Scholar]
|
| 10. |
Ackerman A. Histologic Diagnosis of Inflammatory Skin Diseases. 3rd ed. Philadelphia: Williams & Wilkins; 1997.
[Google Scholar]
|
| 11. |
Sellheyer K, Krahl D. Skin mesenchymal stem cells: Prospects for clinical dermatology. J Am Acad Dermatol 2010;63:859-65.
[Google Scholar]
|
| 12. |
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7.
[Google Scholar]
|
Fulltext Views
3,759
PDF downloads
2,731





