Translate this page into:
Screening for depressive disorders in outpatients with mild to moderate psoriasis: A study from North India
2 Department of Dermatology, Venereology and Leprology, PGIMER, Chandigarh, India
3 Department of Psychiatry, Vardhman Institute of Medical Sciences, Pawapuri, Nalanda, Bihar, India
4 Department of Psychiatry, National Institute of Medical Sciences, Jaipur, Rajasthan, India
Correspondence Address:
Tarun Narang
Department of Dermatology, Venereology and Leprology, PGIMER, Chandigarh - 160 012
India
| How to cite this article: Singh SM, Narang T, Dogra S, Verma AK, Gupta S, Handa S. Screening for depressive disorders in outpatients with mild to moderate psoriasis: A study from North India. Indian J Dermatol Venereol Leprol 2015;81:148-150 |
Abstract
Background: Psoriasis and depressive disorders commonly occur together. Depressive disorders have an impact on the quality of life and the outcome of psoriasis. Aims: The aim of this study was to test the feasibility of using a modification of the Hindi translation of the Patient Health Questionnaire-9 (PHQ-9) as a verbal, clinician administered, short screening questionnaire for detecting depressive disorders. Materials and Methods: One hundred and four out-patients with psoriasis were recruited in the study. In the first stage of the study, socio-demographic data, Psoriasis Area Severity Index (PASI) score, and Dermatological Quality of Life (DLQI) score were recorded. The modified questionnaire was administered by the dermatologist. In the second stage, psychiatric diagnoses were confirmed using the Mini International Neuropsychiatric Interview. Results: The prevalence of depressive disorders was 39.4%. Receiver operating curve (ROC) analysis showed that the questionnaire had a good discriminant ability in detecting depressive disorders (area under curve: 0.81, SE = 0.04, 95% confidence interval = 0.72-0.89). Limitations: The sample size is small and more studies are needed with the screening questions in different languages to validate the findings of the study. Conclusion: The questionnaire can be a useful screening instrument for detecting depressive disorders in patients with psoriasis.INTRODUCTION
Psoriasis is a common, chronic, papulosquamous skin disorder with a complex immune-mediated etiopathogenesis. [1] Depressive disorders are known to be commonly comorbid with psoriasis. [2] The presence of depressive disorders in psoriasis has an adverse bearing on the quality of life. [3],[4] However, the importance of a concomitant depressive disorder on the course and outcome of psoriasis is often underestimated and under-recognized. [5],[6] Thus, there is a need to to rapidly and accurately screen for depressive disorders in this population. We studied the feasibility of using the adaptation of an easily available questionnaire to rapidly screen for depressive disorders in a cohort of outpatients with psoriasis.
Materials and Methods
This study was carried out as a part of a project investigating the prevalence and determinants of common mental disorders in out patients with psoriasis. Other findings are under publication elsewhere. This study was conducted from January to November 2013 in the departments of dermatology and psychiatry at the Post Graduate Institute of Medical Education and Research, Chandigarh, India after obtaining due approval from the Institute Ethics Committee. In the first stage of the study, consecutive patients with chronic plaque psoriasis who were attending the outpatient clinic of the department of dermatology were approached for participation. Written informed consent was obtained from participants in the study. Exclusion criteria included refusal to participate in the study and inability to understand or answer the questions. Patients with an organic brain condition, psoriatic arthritis, erythroderma pustular psoriasis, diabetes, hypertension, any chronic systemic disease, or an underlying psychiatric illness were also excluded. The socio-demographic and clinical details of consenting patients were recorded. Thereafter, the dermatologist asked the participants the first four modified questions of the Patient Health Questionnaire-9 (PHQ-9), [7],[8],[9] a self-administered screening and diagnostic tool for major and other depressive syndromes which has been widely used and validated in various countries and languages. [10] The instrument was modified as follows: (a) The questions were limited to the four most important ones, decided on the basis of clinical experience, instead of nine in the original PHQ-9. (b) instead of asking "how often" they had been bothered by any of the problems listed over the last 2 weeks, the participants were asked, "if" they had been "significantly" bothered by them and the responses were marked as "yes" (Score 1) or "no" (Score 0). The scores were then added to arrive at a final score. The questionnaire as administered is presented in [Figure - 1]. Psoriasis Area Severity Index (PASI) and Dermatological Life Quality Index (DLQI), [11],[12] were also used for evaluation.
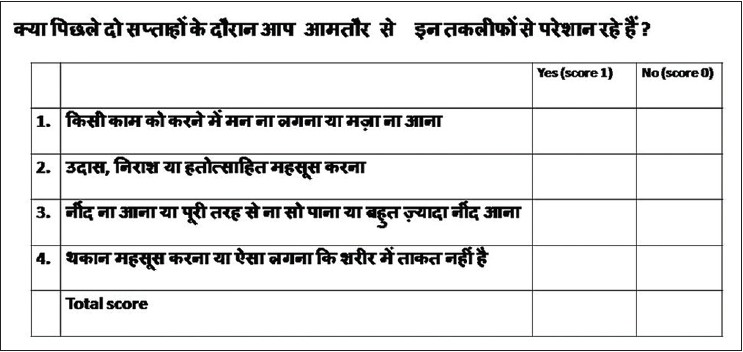 |
| Figure 1: Modified questionnaire used for the study |
The second stage of the study was carried out in the department of psychiatry. In this, the patients were administered the Mini International Neuropsychiatric Interview (MINI) (13) by a psychiatrist who was blinded to the earlier assessment, to generate a psychiatric diagnosis. For the purposes of this part of the study, the diagnosis of depressive disorders included major depressive episode (MDE), current and recurrent, and dysthymia. All data were later analyzed using SPSS software (Version 18.0) for Windows.
RESULTS
A total of 104 participants (79 males and 25 females) consented to be a part of this study. Evaluations with the Psoriasis Area Severity Index (PASI) and Dermatological Life Quality Index (DLQI) indicated that most patients had psoriasis of mild to moderate severity. Mean PASI and DLQI scores with standard deviations of the recruited patients were 6.67 (6.16) and 6.88 (6.15), respectively. The prevalence of depressive disorders was 39.4% (27 of 79 males and 14 of 25 females). The most frequent psychiatric diagnosis was dysthymia which was made in 22 of 27 males and 9 of 14 females. The mean scores and standard deviations on the modified questionnaire in the participants with no psychiatric diagnosis, those diagnosed with dysthymia, and those with major depressive episode were 0.73 (1.24), 2.29 (1.46), and 2.90 (1.28), respectively. Analysis of variance (ANOVA) revealed that the mean scores in the "no psychiatric diagnosis" group were significantly different from those of both the second and third groups (P < 0.05 in both cases). The mean scores in the dysthymia and major depressive episode groups were not found to be significantly different (P = 0.44).
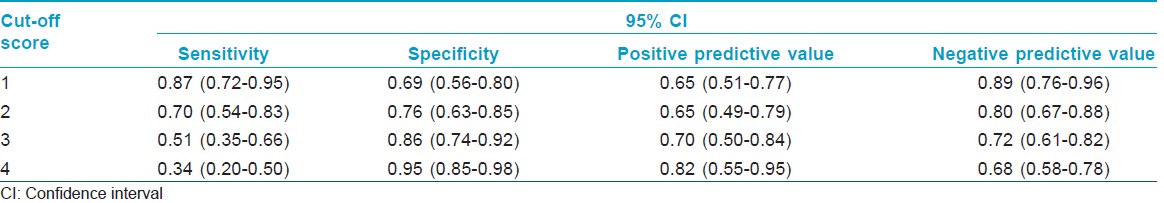
[Table - 1] presents the results of the performance of the modified questionnaire based on the first four questions of PHQ-9 in detecting depressive disorders at different cut-off points. A receiver operating characteristic (ROC) analysis revealed an area under the curve (AUC) of 0.81 (SE = 0.04, 95% confidence interval = 0.72-0.89).
DISCUSSION
A major barrier to the diagnosis of depressive disorders in primary care is the absence of rapidly administered screening instruments [14] and attempts have been made to develop such short screening questionnaires. [15] While screening questionnaires with two or three items have acceptable sensitivity, the specificity is less than desired. [15] Secondly, these questionnaires have been designed to screen for major depression, whereas in our experience, dysthymic disorders are also very common and clinically relevant in the medically ill. Finally, all these questionnaires are in English and are not available in local languages. We attempted to analyze the performance of the first four items of the Hindi translation of PHQ-9 as a rapid, screening instrument for diagnosing depressive disorders. We chose these items for this study because item numbers 1, 2, and 4 are reflective of the major criteria for the diagnosis of major depressive episode as per the ICD-10 and item number 3 inquires into sleep disturbances which are common in patients with depressive disorders, as well as being a criterion for diagnosis of major depressive episode. [16] In our experience, these four problems are most commonly expressed by patients with depressive disorders in our region. Our study population was predominantly composed of subjects with mild to moderate psoriasis in various stages of the disease and treatment. We believe our study population is reflective of the majority of subjects who need to be assessed for depressive disorders. We found that the administration of this questionnaire was useful in screening and took only about a minute to administer. It could be easily administered by the dermatologist and was easily understood by patients. The yes/no answer format reduced ambiguity and the time taken to administer the questionnaire.
The ROC analysis and the area under the curve (AUC) indicated good accuracy of the instrument in the detection of depressive disorders in this population. [17] A cut-off score of 2 or more provided optimum sensitivity and specificity (0.70 and 0.76, respectively). Patients who score more than 2 should be evaluated more thoroughly and managed accordingly. Our results also indicate that the prevalence of dysthymia was more than that of major depressive episode and the severity of the two as measured by the total score on the questionnaire was similar. This is probably because of the chronic stressful nature of psoriasis and the impaired quality of life that leads to chronic depressive states and mild to moderate rather than severe and psychotic major depressive episodes. Screening for depressive disorders in primary care populations should also take cognizance of dysthymic states, which are probably common and cause comparable morbidity, rather than focusing exclusively on major depressive episodes.
We recommend that screening for depressive disorders should be an initial part of a comprehensive, multi-disciplinary management plan for patients with psoriasis.
| 1. |
Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370:263-71.
[Google Scholar]
|
| 2. |
Van Voorhees AS, Fried R. Depression and quality of life in psoriasis. Postgrad Med 2009;121:154-61.
[Google Scholar]
|
| 3. |
Schmitt J, Ford DE. Understanding the relationship between objective disease severity, psoriatic symptoms, illness-related stress, health-related quality of life and depressive symptoms in patients with psoriasis-a structural equations modeling approach. Gen Hosp Psychiatry 2007;29:134-40.
[Google Scholar]
|
| 4. |
Sampogna F, Picardi A, Chren MM, Melchi CF, Pasquini P, Masini C, et al. Association between poorer quality of life and psychiatric morbidity in patients with different dermatological conditions. Psychosom Med 2004;66:620-4.
[Google Scholar]
|
| 5. |
Richards HL, Fortune DG, Weidmann A, Sweeney SK, Griffiths CE. Detection of psychological distress in patients with psoriasis: Low consensus between dermatologist and patient. Br J Dermatol 2004;151:1227-33.
[Google Scholar]
|
| 6. |
Richards HL, Fortune DG, Chong SL, Mason DL, Sweeney SK, Main CJ, et al. Divergent beliefs about psoriasis are associated with increased psychological distress. J Invest Dermatol 2004;123:49-56.
[Google Scholar]
|
| 7. |
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13.
[Google Scholar]
|
| 8. |
Avasthi A, Varma SC, Kulhara P, Nehra R, Grover S, Sharma S. Diagnosis of common mental disorders by using PRIME-MD Patient Health Questionnaire. Indian J Med Res 2008;127:159-64.
[Google Scholar]
|
| 9. |
Spitzer RL, Williams JB, Kroenke K. PHQ-9. New York: Pfizer Inc; 2001. Available from: http://www.phqscreeners.com [Last accessed on 2014 Sep 10].
[Google Scholar]
|
| 10. |
Spitzer RL, Williams JB, Kroenke K. PHQ-9. New York: Pfizer Inc; 2001. Available from: http://www.phqscreeners.com/articles/01_Bibliography%20by%20author.pdf. [Last accessed on 2014 Sep 10].
[Google Scholar]
|
| 11. |
Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician′s Global Assessment. J Am Acad Dermatol 2004;51:563-9.
[Google Scholar]
|
| 12. |
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-6.
[Google Scholar]
|
| 13. |
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59 Suppl 20:22-33.
[Google Scholar]
|
| 14. |
Andersen SM, Harthorn BH. The recognition, diagnosis, and treatment of mental disorders by primary care physicians. Med Care 1989;27:869-86.
[Google Scholar]
|
| 15. |
Mitchell AJ, Coyne JC. Do ultra-short screening instruments accurately detect depression in primary care? A pooled analysis and meta-analysis of 22 studies. Br J Gen Pract 2007;57:144-51.
[Google Scholar]
|
| 16. |
World Health Organization. International Classification of Diseases-10. Geneva: World Health Organization; 1994.
[Google Scholar]
|
| 17. |
Metz CE. Basic principles of ROC analysis. Semin Nucl Med 1978;8:283-98.
[Google Scholar]
|
Fulltext Views
3,117
PDF downloads
2,486





