Translate this page into:
Selenium toxicity: A rare diagnosis
Correspondence Address:
Puneet Agarwal
397, Shree Gopal Nagar, Gopalpura Bypass, Jaipur, Rajasthan
India
| How to cite this article: Agarwal P, Sharma S, Agarwal US. Selenium toxicity: A rare diagnosis. Indian J Dermatol Venereol Leprol 2016;82:690-693 |
Sir,
While trace elements are essential for normal body growth and regulation, excess intake of these elements can lead to toxic effects.[1] Selenium is an essential trace element which is a constituent of more than two dozen selenoproteins that play a critical role in reproduction, thyroid hormone metabolism, deoxyribonucleic acid synthesis and protection from oxidative damage and infection.[2] However, it is known to be toxic with a narrow interval separating deficiency and toxicity. According to the World Health Organization, selenium toxicity is prevalent in South Dakota (USA), Venezuela, and China.[1] In India, selenium toxicity has been reported in Hoshiarpur and Nawansahar districts of Punjab.
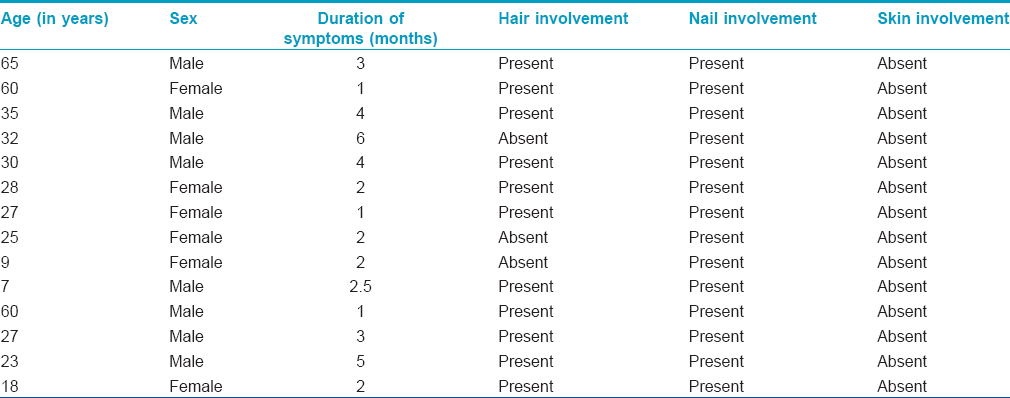
A 35-year-old man presented at SMS Medical College and Hospital, Jaipur with a four-month history of dystrophy of all nails and a one-month history of hair loss and fragility of hair all over the body. Initially, white spots and longitudinal red streaks appeared on the nail surface followed by increased brittleness and breaks in the nail plate. [Figure - 1]a and [Figure - 1]b. The new nails that grew subsequently were also dystrophic. The thumb nail was the first to be affected and later all twenty nails were involved [Figure - 2]. In some of the fingernails, fluid effused from the nail folds and there was pain at the fingertips. Within 2-3 months he noticed increased hair fragility and loss of scalp, beard, axillary, pubic and body hair [Figure - 3]. There was no significant history of any drug intake or chronic systemic disease. There were similar complaints in eleven members of his family and four members of a neighboring family [Table - 1]. On examination, there was involvement of all the finger and toe nails with varying severity in these individuals. There was patchy erythronychia with transverse, subtotal or distal leukonychia, onycholysis and roughening of the nail plate. Hair on the scalp, eyebrows, beard and mustache were dry, brittle and broke easily on slight pulling. Hair density was also decreased on the entire scalp, most pronounced on the occipital area. There was no mucosal involvement. General physical and systemic examinations were also unremarkable. Potassium hydroxide preparation and fungal culture of nail clippings were negative. Hair microscopy showed pigmented, shrunken anagen hair bulbs. Hair shafts were normal with no beading, twisting, nodes or fractures. Nail biopsy showed hyperkeratosis and irregular acanthosis with intra-corneal neutrophilic abscess. An intra-epidermal cleft was seen, filled with red blood cells and acute and chronic inflammatory cells. The dermis showed dense perivascular infiltration by acute and chronic inflammatory cells. All routine blood investigations were within normal limits.
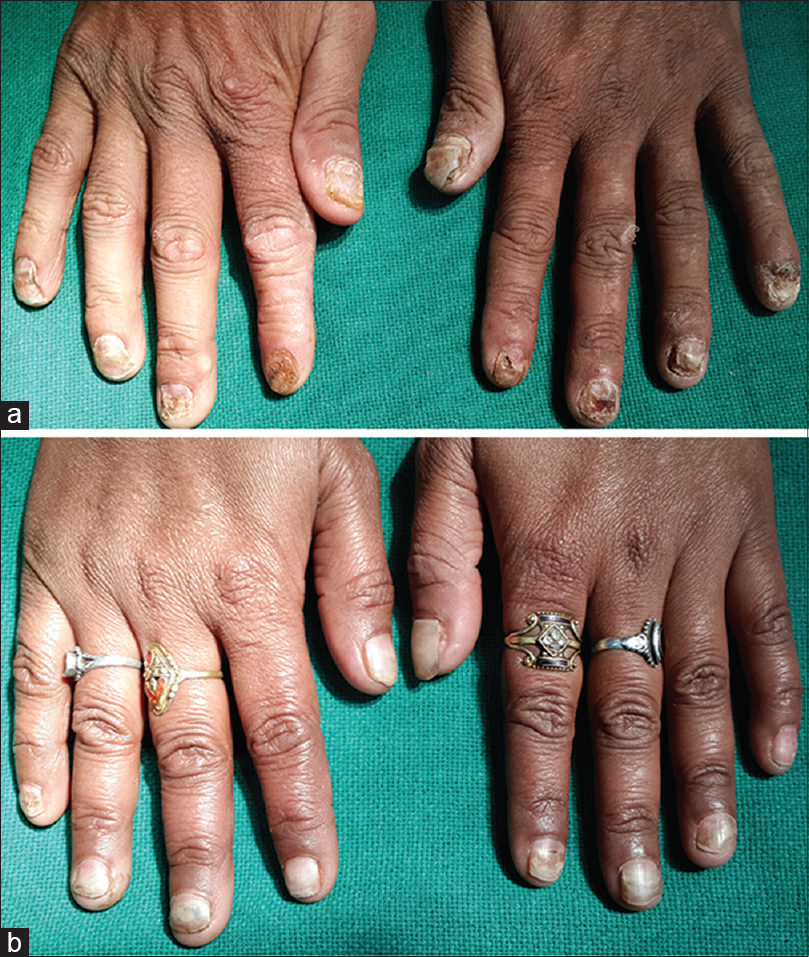 |
| Figure 1: (a) Dystrophy of fingernails, (b) dystrophy of fingernails in another patient |
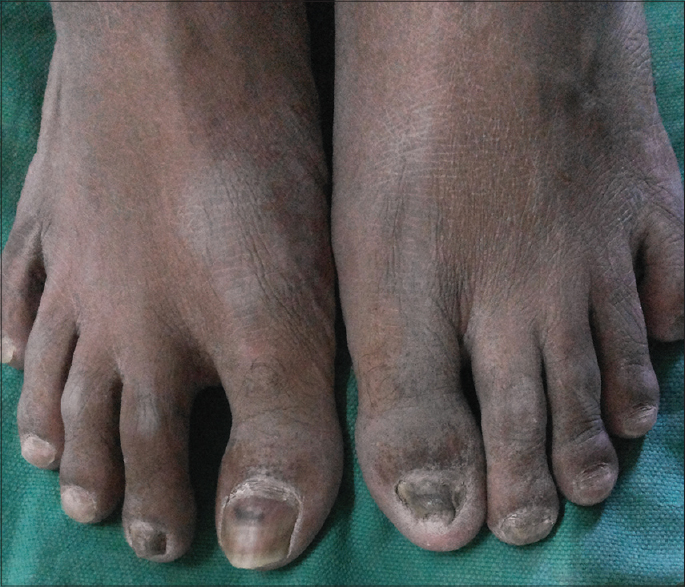 |
| Figure 2: Dystrophy of toenails |
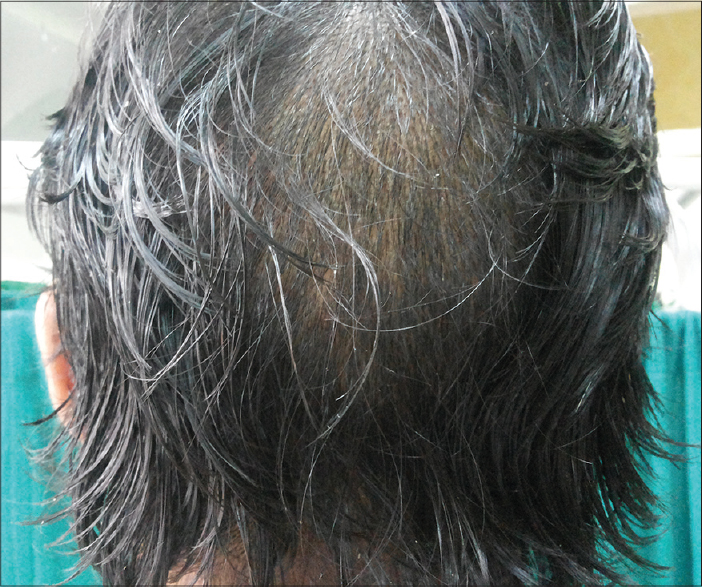 |
| Figure 3: Patchy loss of hair over scalp |
On further evaluation, no abnormality was detected on electrocardiography, however, nerve conduction studies revealed signs of peripheral motor neuropathy affecting both the common peroneal nerves. Magnetic resonance angiography of the hand (to rule out microvascular involvement) was normal and computed tomography scan of the hand showed osteochondritis of the carpal bone.
Abrupt onset of hair loss and nail dystrophy and the presence of symptoms in the whole family along with a neighboring family made us suspect chronic poisoning due to some organic or inorganic material. Samples were taken from the hair and nails of the most severely affected patient and were sent to a laboratory for analysis.
Inductively coupled plasma mass spectrometry revealed an excessive amount of selenium in the samples. The concentrations of selenium detected in the nail and hair were 27 µg/g and 35 µg/g respectively. The corresponding normal values are 0.809 µg/g and 0.36 µg/g, respectively for nail and hair.[3],[4] Thus, the amount of selenium detected was almost 30 times higher in nails and almost 100 times higher in hair. Since the signs of poisoning were limited to only two families in the area, the potential sources were either polluted water supply or food material. It was found that both families were using wheat grain from the same source. Thus, the suspected wheat grain sample along with a control wheat grain sample (with no symptoms on consumption) were sent to the laboratory for analysis. Furthermore, a water sample was sent for analysis. The selenium concentration in the control wheat grain sample was 0.43 mg/kg, whereas it was 152.82 mg/kg in the suspected wheat grain sample. The reported normal value for selenium in wheat grains is 0.61 ± 0.006 mg/kg.[4] Thus, the level detected in the suspected wheat grains was almost 250 times higher than normal. Selenium was not detectable in the water sample.
The patients recovered remarkably, with growth of healthy nails and hair, after they stopped consumption of the toxic wheat.
Selenium is an essential element and its recommended daily allowance varies from 55 to 70 μg/day. The major source is dietary; it is present mostly bound to animal and plant proteins. The food sources are meats and seafood (0.3–0.5 mg/kg), cereals (0.1–10 mg/kg), vegetables and fruits (<0.01 mg/kg). The selenium content in crops depends on the water soluble selenium present in soil and the amount in animals depends on their feed. Water per se, due to its low selenium content, is an unlikely source of selenium toxicity except in areas with an excess of selenium in the soil.[5] The level of selenium in most urban air ranges from 0.1 to 10 ng/m [3], but higher levels may be found in certain areas such as near copper smelters.[1]
Most water-soluble inorganic and organic selenium compounds present in food are absorbed across the gastrointestinal tract (80–95%).[6] After absorption, selenium is cleared by the liver and then transported by a specific transporter, selenoprotein P, to all organs with the highest concentrations occurring in the kidney, liver, spleen, testes and skeletal muscle.[7],[8] The mechanisms of selenium toxicity at the cellular or molecular level are not yet fully understood. However, it is believed that it can interact with glutathione to form reactive selenotrisulfides and generate toxic superoxide and hydrogen peroxide species, oxidizing cell membranes and macromolecules, thereby damaging cell integrity and resulting in necrosis or apoptosis.[9] In a study in which human cells were treated with selenium, it was observed that selenium at higher levels has a cytotoxic effect with depletion of lipids and proteins and DNA damage.[10] Stewart et al. reported significant DNA-adduct formation in mouse keratinocytes (BALB/c MK-2) treated with 5 mg/L Se iv as a consequence of oxidation which is thought to directly arise from the generation of the hydroxyl (−OH) radicals.[11]
Very low selenium status in humans has been associated with a juvenile, multifocal myocarditis called Keshan disease and a chondrodystrophy called Kaschin–Beck disease.[12],[13],[14] Chronic selenium toxicity leads to loss of hair and nails. Hair breaks at the level of the scalp with slight trauma, such as scratching, while the follicles remain intact. Hair can be lost from any body site. A rash on the scalp with severe itching has also been reported. Nails become brittle and are shed and the new nail is also dystrophic. Skin lesions have been reported, mainly on the limbs, i.e., dorsum of hands and feet, the outer side of legs and thighs, the forearms and the back of the neck. Affected skin becomes red and swollen and then blistered and ulcerated. Reddish pigmentation of the skin usually remains and gooseflesh may be left on the neck and thighs. Dental anomalies such as tooth decay and mottling might also be seen. Abnormalities of the nervous system such as peripheral anesthesia, “pins and needles,” acroparesthesia, pain in the extremities, hyperreflexia of the tendons and motor disturbances are other features. Decreases in prothrombin time and in the concentration of glutathione have also been reported.[3],[4],[14] The only effective treatment is discontinuation of the source of selenium, i.e., either contaminated food material or water.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
WHO. Selenium in drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality. (WHO/HSE/WSH/10.01/14). WHO; 2011.
[Google Scholar]
|
| 2. |
Sunde RA. Selenium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Modern Nutrition in Health & Disease. 11th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2012. p. 225-37.
[Google Scholar]
|
| 3. |
Yang G, Zhou R, Yin S, Gu L, Yan B, Liu Y, et al. Studies of safe maximal daily dietary selenium intake in a seleniferous area in China. I. Selenium intake and tissue selenium levels of the inhabitants. J Trace Elem Electrolytes Health Dis 1989;3:77-87.
[Google Scholar]
|
| 4. |
Yang GQ, Wang SZ, Zhou RH, Sun SZ. Endemic selenium intoxication of humans in China. Am J Clin Nutr 1983;37:872-81.
[Google Scholar]
|
| 5. |
Available from: http://www.ods.od.nih.gov/factsheets/Selenium-HealthProfessional/. [Last accessed on 2015 Jan 03].
[Google Scholar]
|
| 6. |
Bopp BA, Sonders RC, Kesterson JW. Metabolic fate of selected selenium compounds in laboratory animals and man. Drug Metab Rev 1982;13:271-318.
[Google Scholar]
|
| 7. |
Brown DG, Burk RF. Selenium retention in tissues and sperm of rats fed a Torula yeast diet. J Nutr 1973;103:102-8.
[Google Scholar]
|
| 8. |
Thomassen Y, Aaseth J. Selenium in human tissues. In: Ihnat M, editor. Occurrence and Distribution of Selenium. Boca Raton, FL: CRC Press; 1986.
[Google Scholar]
|
| 9. |
Spallholz JE. On the nature of selenium toxicity and carcinostatic activity. Free Radic Biol Med 1994;17:45-64.
[Google Scholar]
|
| 10. |
Llabjani V, Hoti V, Pouran HM, Martin FL, Zhang H. Bimodal responses of cells to trace elements: Insights into their mechanism of action using a biospectroscopy approach. Chemosphere 2014;112:377-84.
[Google Scholar]
|
| 11. |
Stewart MS, Spallholz JE, Neldner KH, Pence BC. Selenium compounds have disparate abilities to impose oxidative stress and induce apoptosis. Mar Environ Res 1999;26:42-8.
[Google Scholar]
|
| 12. |
Högberg J, Alexander J. Selenium. In: Friberg L, Nordberg GF, Vouk VB, editors. Handbook on the Toxicology of Metals. 2nd ed., Vol. 2. Amsterdam: Elsevier; 1986. p. 482-520.
[Google Scholar]
|
| 13. |
IPCS. Selenium. (Environmental Health Criteria, No. 58). International Programme on Chemical Safety. Geneva: World Health Organization; 1987.
[Google Scholar]
|
| 14. |
FAO/WHO. Vitamin and Mineral Requirements in Human Nutrition. Report of a Joint FAO/WHO Expert Consultation, Bangkok, Thailand, 21-30 September 1998. 2nd ed. Geneva: World Health Organization; 2004. Available from: http://www.ods.od.nih.gov/factsheets/Selenium-HealthProfessional/.
[Google Scholar]
|
Fulltext Views
12,360
PDF downloads
2,394





