Translate this page into:
Self-perceived emotional side effects of systemic corticosteroid therapy in dermatology patients
2 Department of Dermatology, Venereology and Leprology, PGIMER, Chandigarh, India
Correspondence Address:
Tarun Narang
Department of Dermatology, Venereology and Leprology, PGIMER, Chandigarh - 160 012
India
| How to cite this article: Singh SM, Narang T, Dogra S, Handa S. Self-perceived emotional side effects of systemic corticosteroid therapy in dermatology patients. Indian J Dermatol Venereol Leprol 2015;81:655 |
Sir,
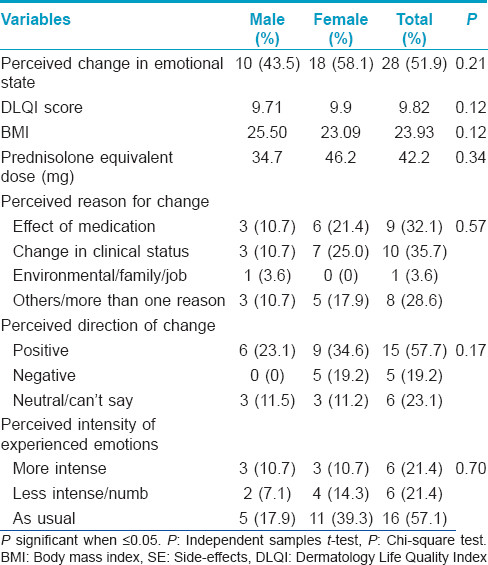
Corticosteroid (CS) therapy is known to be associated with a variety of neuropsychiatric and cognitive side effects of varying severity.[1] Data exist for the more severe, syndromic manifestations of the side effects spectrum even though milder forms are likely to be more common.[2] Our study aims to provide some preliminary data regarding the prevalence and correlates of self-perceived emotional side effects in patients with dermatological conditions on corticosteroids therapy. This study was carried out at the Postgraduate Institute of Medical Education and Research, Chandigarh, a tertiary care hospital in north India, after approval by the institute ethics committee. Consecutive, consenting adult patients in the dermatology outpatient service on systemic corticosteroids for at least 4 weeks were recruited. The quality of life was recorded using the dermatology life quality index (DLQI).[3] Each participant completed a self-administered questionnaire [Figure - 1] regarding their current emotional state and any perceived relationship with steroid therapy. The items in the questionnaire were included on the basis of clinical experience. The questionnaire included inquiries about whether any change in emotional state was perceived following corticosteroids, perceived reasons for any change, whether the change was perceived as positive or negative and the perception of any change in the intensity of experienced emotions. Twenty three men and 31 women participated in the study. The mean age was 41.47 (±16.43) and 42.29 (±13.06) years for men and women, respectively. The two groups were comparable in other aspects such as years of completed education (P = 0.06), duration of illness (mean = 57.63 and 56.88 months, P = 0.32), duration of treatment (mean = 17.01 and 31.01 months, P = 0.13), Dermatology Life Quality Index (DLQI) scores (mean = 8.00 and 5.02, P = 0.36) and body mass index (BMI) (mean = 24.45 and 23.15, P = 0.73). A majority of participants were on oral prednisolone (38; 70.4%) followed by dexamethasone (7; 13.0%). The most common route of administration was oral (49; 90.7%) followed by parenteral (intravenous).[4] Twenty-seven (50%) of the participants had been on daily corticosteroid therapy for <6 months, followed by those on daily long-term therapy (>6 months) (19; 35.2%) and dexamethasone pulse for pemphigus vulgaris (5; 9.3%). Data in this regard were missing for 3 (5.6%) participants. The majority of the patients had pemphigus vulgaris (14; 25.9%), airborne contact dermatitis (11; 20.4%) and endogenous eczema (6; 11.1%). The average prednisolone equivalent dose was 40.37 mg and was not significantly different between men and women (P = 0.22). [Table - 1] presents the details of those who perceived emotional side effects associated with steroid therapy. We also compared those who perceived emotional change versus those who did not. Those who perceived emotional change had a greater body mass index (23.93 vs. 23.47, P = 0.01) but did not significantly differ on other variables. Subanalyses for men and women separately revealed similar results. Those who attributed their emotional change to the effect of medications experienced significantly more positive emotions than those who attributed it to other reasons (P = 0.01) and also had significantly lower body mass index scores (21.42 vs. 25.01, P = 0.004) but did not perceive any change in the intensity of experienced emotions (P = 0.48). Corticosteroid - associated neuropsychiatric side effects tend to be dose related, occur early in the course of treatment and in severe cases may necessitate discontinuation of steroids. All manner of psychiatric syndromes from cognitive dysfunction, delirium, psychotic, affective and anxiety disorders are known to occur with the use of corticosteroids. Manic symptomatology seems to be the most common followed by depressive symptoms.[2] Data on emotional side effects exist in the context of multidimensional investigations and rating scale development.[4],[5] Emotional side effects reported included neuropsychiatric disorders or “feeling grumpy,” “mood swings” and “feeling irritated” were dose related and as high as 60%. The transition and relationship of non-syndromic emotional side effects to syndromic psychiatric side effects remains obscure. Our study population consisted of dermatological patients on generally lower, oral doses of corticosteroid therapy in disorders that are not usually associated with neuropsychiatric symptoms. We found that emotional side effects are commonly perceived in association with corticosteroid therapy in dermatological patients on low to moderate doses. This change is attributed to positive or negative change in clinical status and other environmental factors. The stress of long standing illness and treatment may also contribute. A significant minority attribute this change exclusively to the effect of corticosteroid. This group seems to experience more positive emotional side effects and have lower body mass index. Lower body mass index may indicate greater bioavailability of steroid at similar doses leading to the development of side effects. Our results suggest that there may be a subgroup of individuals that is sensitive to developing emotional side effects in the context of corticosteroid therapy. The nature of this sensitivity is likely to be idiosyncratic but dose related with respect to the severity of presentation from non-specific emotional side effects to diagnosable psychiatric disorders. The limitations of the study are the lack of a control group, small sample size and cross-sectional rather than the longitudinal nature of assessment.
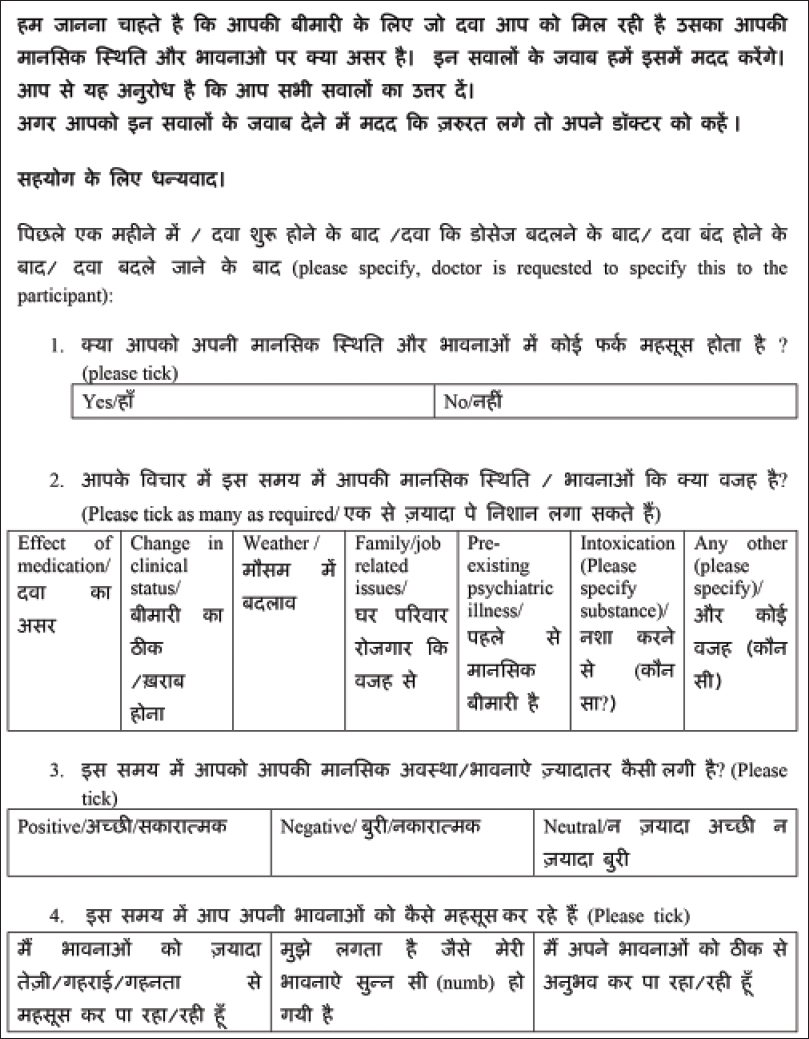 |
| Figure 1: Patient rated questionnaire used for the study |
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Dubovsky AN, Arvikar S, Stern TA, Axelrod L. The neuropsychiatric complications of glucocorticoid use: Steroid psychosis revisited. Psychosomatics 2012;53:103-15.
[Google Scholar]
|
| 2. |
Kenna HA, Poon AW, de Los Angeles CP, Koran LM. Psychiatric complications of treatment with corticosteroids: Review with case report. Psychiatry Clin Neurosci 2011;65:549-60.
[Google Scholar]
|
| 3. |
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – A simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-6.
[Google Scholar]
|
| 4. |
Fardet L, Flahault A, Kettaneh A, Tiev KP, Généreau T, Tolédano C, et al. Corticosteroid-induced clinical adverse events: Frequency, risk factors and patient's opinion. Br J Dermatol 2007;157:142-8.
[Google Scholar]
|
| 5. |
Foster JM, van Sonderen E, Lee AJ, Sanderman R, Dijkstra A, Postma DS, et al. A self-rating scale for patient-perceived side effects of inhaled corticosteroids. Respir Res 2006;7:131.
[Google Scholar]
|
Fulltext Views
2,030
PDF downloads
1,407





