Translate this page into:
Severe cutaneous adverse reactions
Correspondence Address:
Sanjiv Grover
Department of Dermatology, Air Force Hospital, Jorhat, Assam - 785005
India
| How to cite this article: Grover S. Severe cutaneous adverse reactions. Indian J Dermatol Venereol Leprol 2011;77:3-6 |
Primum non nocere ("first of all be sure you do no harm")
Hippocrates (460-370 BC)
Since time immemorial, medications of various kinds have been used by physicians with the noble intention of curing the sufferer of his ailments. Yet, paradoxically, this well-meaning intention may become the nemesis of many a sufferer and this Hippocratic principle may willy-nilly be defeated. While adverse drug reactions (ADRs) are as old as Medicine itself, cutaneous ADRs may be severe enough to threaten life.
Cutaneous ADRs
ADRs are reportedly responsible for up to 7% of hospital admissions, and cutaneous ADRs alone contribute to 2-3% of the overall hospital admissions. [1],[2] Up to 30-45% of the ADRs are reportedly cutaneous in nature, 2% of which may be severe and few may even end in fatalities. [3],[4] This adds up to a significant proportion of patients at risk who have to be dealt with effectively.
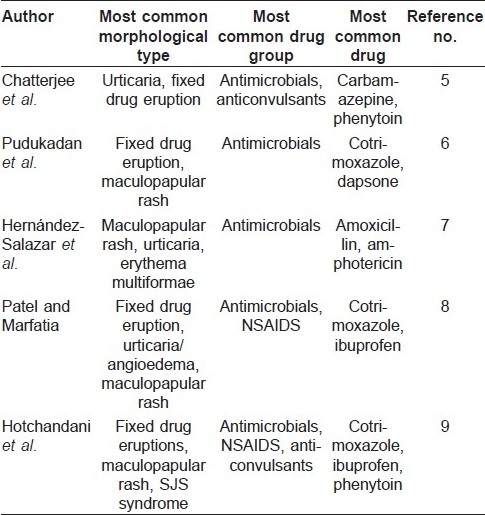
The most common morphological types of cutaneous ADRs range from maculopapular, urticaria/angioedema to fixed drug eruptions, and the common incriminating drug groups remain antimicrobials, anticonvulsants and non-steroid anti-inflammatory drugs (NSAIDS). [5],[6],[7],[8],[9] In spite of remarkable similarities in the findings, differences could well reflect variations in the prescribing patterns of drugs across these study groups.
As these ADRs could be seen across a wide spectrum of classes of drugs, clinical diagnosis may be difficult. Diagnosis could be further confounded by history of multiple drug intake, viral fever or cutaneous manifestations of internal diseases. Roujeau′s criteria [10] attempted to simplify defining cutaneous ADRs, viz (a) other causes for the eruption as viral exanthema should be excluded, (b) a temporal relationship between the drug and onset of rash should exist, (c) improvement should be noted following drug cessation, (d) reactivation upon challenge should be noted and (e) cutaneous reaction is known to be associated with the drug. Clinical cases of morbilliform maculopapular ADRs have been pathologically correlated with findings of superficial dermal infiltrates of lymphocytes, eosinophils and neutrophils with or without interface changes. [11]
Certain risk factors for ADRs are (a) patient related, viz age of patients, female sex, viral infection, genetic variations in the metabolism of the drug and human leucocyte antigen (HLA) association and (b) drug related, viz number of drugs taken, route of administration, duration of intake, dose and variation in metabolism. [12],[13]
Severe Cutaneous ADRs
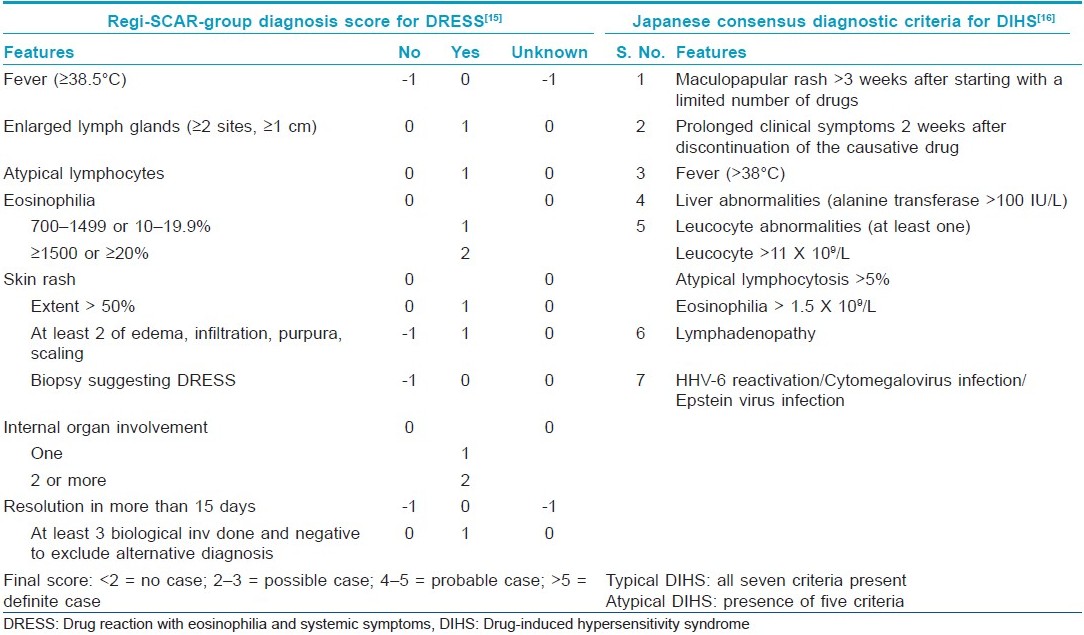
Cutaneous ADRs in the form of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug hypersensitivity reactions (DHR) or drug reaction with eosinophilia and systemic symptoms (DRESS) or drug-induced hypersensitivity syndrome (DIHS) and acute generalized exanthematous pustulosis (AGEP) could be severe and life threatening. The term severe cutaneous adverse reactions (SCAR) was proposed for such conditions, as they were (a) severe, (b) unpredictable and (c) drug induced. [8] Over 200 drugs have been implicated in the literature that can cause SCARs. Causality of the ADR could be measured using the WHO-UMC causality assessment system, which grades the assessment across a spectrum from "certain" to "unlikely" to "unclassified" and "unclassifiable." [14] Clinical criteria for diagnosis and scoring of DRESS, [15] DIHS, [16] AGEP [17] and TEN [18] have been devised and reported in the literature [Table - 1] and [Table - 2].

DHRs may comprise up to one third of all ADRs. [19] They are peculiar, in that they (a) cannot be predicted, (b) do not show any relationship to dose, (c) effect a minority of patients and (d) cannot be reproduced in animal models. [20] Despite various nomenclatures, it is established that DHRs start later, last longer, are associated with visceral abnormalities and may require longer therapy as compared with other drug "rashes." Exfoliative dermatitis, due to psoriasis, eczema or lymphoma, angioimmunoblastic lymphadenopathy, viral exanthem and vasculitis count among the differential diagnoses of DHRs. [21] AGEP is characterized by numerous non-follicular pustules on widespread edematous erythema shortly following antibiotic administration and may need to be differentiated from pustular psoriasis. DRESS is a unique drug rash (begins as morbilliform eruption, later become edematous, may evolve into vesicles and tense bullae like TEN, erythroderma or purpuric lesions) as stoppage of the drug, although required, may not lead to subsidence of the rash; rather, the rash progresses further and facial edema is a hallmark feature. It may involve the occurrence of multiorgan involvement, requirement for a long course of steroids as compared with other SCARs and occurrence of relapse if steroids are tapered and stopped fast. As compared with SJS and TEN, lymphocytosis and eosinophilia are the feature of DRESS rather than lymphocytopenia, thrombocytopenia and neutropenia as seen in SJS/TEN.
SJS and TEN are associated with severe morbidity and mortality. While the mortality rate in SJS is 5-10%, that in TEN is reportedly 25-30%. A causal relationship with drugs is found in two-third of all cases. Skin pain, positive Nikolsky′s sign and epidermolysis have been considered to be the most important "danger signs" of an impending SCAR. [22] Dermal cell apoptosis, triggered by Fas and Fas ligand, tumor necrosis factor - a (TNF-a), TNF-a-related apoptosis-inducing ligand and granzyme B, is the predominant factor in the etiology of SJS/TEN. Prompt withdrawal of the incriminating drug, limiting systemic corticosteroids to the first 2 days, commencing cyclosporine or intravenous immunoglobulin (IVIg) within the first 4 days and supportive treatment form the principles of management of these conditions. [23]
Genetic Link and Diagnostic Tools
Cutting-edge research in delineating the genetic markers for SCAR has revealed strong associations between human leukocyte antigen subtypes and certain SCARs. SCAR due to allopurinol has been linked to HLA-BFNx015801 and that due to carbamazepine has been associated with HLA-BFNx011502. [24] Skin testing (patch test, prick test, intradermal test) with the suspected compound has been reported to be helpful in determining the cause of cutaneous ADRs. Criteria for determining the imputability of drug, [25] guidelines for patch testing [26] and guidelines for intradermal testing for the incriminating drug [27] have already been well described in the literature and serve as valuable tools in establishing the definitive cause of the cutaneous ADR. The success of these skin drug tests depends on the drug tested, its concentration, its volume, the method used, choice of the vehicle and the clinical features of the ADR. Yet, it is important to remember that skin testing is negative in 30-50% of the patients. Conversely, false-positive results may compel the treating physician to think hard about the relevance and specificity of the skin test results. [28] Appropriate negative-control patients are recommended to be used in order to avoid false-positive results. Meanwhile, low sensitivity of the patch tests in SJS/TEN has also been reported. [29] The oral rechallenge test, although a tool to establish the drug-rash relationship, should be avoided in SCARs.
Management
Multiple organ system involvement in SCARs necessitates a multispecialty approach. In the absence of effective evidence-based treatment protocols and with no consensus on treatment of SCARs, especially with the use of systemic steroids, controversies still exist in the management of SCARs. [30] While newer drugs like lamotrigine, nevirapine and imatinib add to the burgeoning list of drugs incriminated in SCAR, newer therapies like infliximab are emerging as effective therapeutic options in its management. [31],[32] The ongoing Regi-SCAR study, building on the lessons learnt from the SCAR and Euro-SCAR studies, will surely help the future generations in surmounting this formidable and challenging task of managing potentially fatal cases. [33]
| 1. |
Wiffen P, Gill M, Edwards J, Moore A. Adverse drug reactions in hospital patients. A systematic review of the prospective and retrospective studies. Bandolier Extra 2002;2:1-16.
[Google Scholar]
|
| 2. |
Gruchalla R. Understanding drug allergies. J Allergy Clin Immunol 2000;105:637-44.
[Google Scholar]
|
| 3. |
Naldi L, Conforti A, Venegoni M, Troncon MG, Caputi A, Ghiotto E, et al. Cutaneous reactions to drugs. An analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol 1999;48 : 839-46.
[Google Scholar]
|
| 4. |
Ajayi FO, Sun H, Perry J. Adverse drug reactions: A review of relevant factors. J Clin Pharmacol 2000;40:1093-101.
[Google Scholar]
|
| 5. |
Chatterjee S, Ghosh AP, Barbhuiya J, Dey SK. Adverse cutaneous drug reactions: A one year survey at a dermatology outpatient clinic of a tertiary care hospital. Indian J Pharmacol 2006;38:429-31.
[Google Scholar]
|
| 6. |
Pudukadan D, Thappa DM. Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care centre in South India. Indian J Dermatol Venereol Leprol 2004;70:20-4.
[Google Scholar]
|
| 7. |
Hernαndez-Salazar A, Rosales SP, Rangel-Frausto S, Criollo E, Archer-Dubon C, Orozco-Topete R. Epidemiology of adverse cutaneous drug reactions. A prospective study in hospitalized patients. Arch Med Res 2006;37:899-902.
[Google Scholar]
|
| 8. |
Patel RM, Marfatia YS. Clinical study of cutaneous drug eruptions in 200 patients. Indian J Dermatol Venereol Leprol 2008;74:80.
[Google Scholar]
|
| 9. |
Hotchandani SC, Bhatt JD, Shah MK. A prospective analysis of drug-induced acute cutaneous reactions reported in patients at a tertiary care hospital. Indian J Pharmacol 2010;42:118-9.
[Google Scholar]
|
| 10. |
Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331 : 1272-85.
[Google Scholar]
|
| 11. |
Gerson D, Sriganeshan V, Alexis JB. Cutaneous drug eruptions: A five year experience. J Am Acad Dermatol 2008;59:995-9.
[Google Scholar]
|
| 12. |
Nayak S, Acharjya B. Adverse cutaneous drug reaction. Indian J Dermatol 2008;53:2-8.
[Google Scholar]
|
| 13. |
Svensson CK, Cowen EW, Gaspari AA. Cutaneous Drug Reactions. Pharmacol Rev 2000;53:357-9.
[Google Scholar]
|
| 14. |
The use of the WHO-UMC system for standardized case causality assessment. http://www.who-umc.org/graphics/4409.pdf. [Last accessed on 2010 October 24].
[Google Scholar]
|
| 15. |
Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol 2007;156:609-11.
[Google Scholar]
|
| 16. |
Shiohara T, Ijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol 2007;156:1083-4.
[Google Scholar]
|
| 17. |
Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. J Cutan Pathol 2001;28:113-9.
[Google Scholar]
|
| 18. |
Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: A Severity-of-Illness Score for Toxic Epidermal Necrolysis. J Invest Dermatol 2000;115:149-53.
[Google Scholar]
|
| 19. |
Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Current Opinion Allergy Clin Immunol 2005;5:309-16.
[Google Scholar]
|
| 20. |
Park BK, Pirmohamed M, Kitteringham NR. The role of drug disposition in drug hypersensitivity: A chemical, molecular and clinical perspective. Chem Res Toxicol 1998;11:969-88.
[Google Scholar]
|
| 21. |
Bachot N, Roujeau JC. Differential Diagnosis of Severe Cutaneous Drug Eruptions. Am J Clin Dermatol 2003;8:561-72.
[Google Scholar]
|
| 22. |
Bircher AJ. Symptoms and dangers signs in drug hypersensitivity. Toxicol 2005;209:201-7.
[Google Scholar]
|
| 23. |
Chia FL, Leong KP. Severe cutaneous adverse reactions to drugs. Curr Opin Allergy Clin Immunol 2007;7:304-9.
[Google Scholar]
|
| 24. |
Roujeau JC, Allanore L, Liss Y, Mockenhaupt M. Severe Cutaneous Adverse Reactions to Drugs (SCAR): Definitions, Diagnostic Criteria, Genetic Predisposition. Dermatol Sinica 2009;12:203-9.
[Google Scholar]
|
| 25. |
Moore N, Paux G, Begaud B, Biour M, Loupi E, Boismare F, et al. Adverse drug reaction monitoring: Doing it the French way. Lancet 1985;2:1056-8.
[Google Scholar]
|
| 26. |
Barbaud A, Gonηalo M, Bruynzeel D, Bircher A. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis 2001;45:321-8.
[Google Scholar]
|
| 27. |
Barbaud A, Reichert-Penetrat S, Trechot P, Jacquin-Petit MA, Ehlinger A, Noirez V, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol 1998;139:49-58.
[Google Scholar]
|
| 28. |
Barbaud A, Trechot P, Reichert-Penetrat S, Commun N, Schmutz JL. Relevance of skin tests with drugs in investigating cutaneous adverse drug reactions. Contact Dermatitis 2001;45:265-8.
[Google Scholar]
|
| 29. |
Wolkenstein P, Chosidow O, Flιchet ML, Robbiola O, Paul M, Dumι L, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis 1996;35:234-6.
[Google Scholar]
|
| 30. |
Wolf R, Davidovici B. Severe cutaneous adverse drug reactions: who should treat, where and how? Facts and controversies. Clin Dermatol 2010;28:344-8.
[Google Scholar]
|
| 31. |
Ugurel S, Hildenbrand R, Dippel E, Hochhaus A, Schadendorf D. Dose-dependent severe cutaneous reactions to imatinib. Br J Cancer 2003;88:1157-9.
[Google Scholar]
|
| 32. |
Hunger RE, Hunziker T, Buettiker U, Braathen LR, Yawalkar N. Rapid resolution of toxic epidermal necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol 2005;116:923-4.
[Google Scholar]
|
| 33. |
Kelly JP, Auquier A, Rzany B, Naldi L, Bastuji-Garin S, Correia O, et al. An international collaborative case-control study of severe cutaneous adverse reactions (SCAR). Design and methods. J Clin Epidemiol 1995;48:1099-108.
[Google Scholar]
|
Fulltext Views
10,822
PDF downloads
4,418





