Translate this page into:
Spectrum of purpura fulminans: Report of three classical prototypes and review of management strategies
Correspondence Address:
Ankur Talwar
Department of Dermatology, Kempegowda Institute of Medical Sciences Hospital, KR Road, VV Puram, Bangalore
India
| How to cite this article: Talwar A, Kumar S, Gopal M G, Nandini A S. Spectrum of purpura fulminans: Report of three classical prototypes and review of management strategies. Indian J Dermatol Venereol Leprol 2012;78:228 |
Abstract
Purpura fulminans is a rare syndrome of intravascular thrombosis and hemorrhagic infarction of the skin that is rapidly progressive and is accompanied by vascular collapse and disseminated intravascular coagulation. It usually occurs in children, but this syndrome has also been noted in adults. The three forms of this disease are classified by the triggering mechanisms. We describe three classical cases of purpura fulminans of the three classical prototypes treated at our center and their varied clinical outcomes. We also describe a case of acute infectious purpura fulminans secondary to systemic leptospirosis which to our best knowledge is the first reported case in world literature. The various treatment options for purpura fulminans have also been reviewed.Introduction
Purpura fulminans (PF) is an hemorrhagic potentially disabling and life-threatening skin disorder. Features include tissue necrosis, small vessel thrombosis, and disseminated intravascular coagulation. Three distinct categories can be identified: Inherited abnormalities of protein C or other coagulation systems, acute infectious PF, and idiopathic. [1] Many cases have been reported in the pediatric literature, and about 90% of them have been fatal. [2] With the advent of new therapeutic approaches, the survival rate has increased. We describe three cases of purpura fulminans of the three typical prototypes and their varied clinical course and outcome. We have also reviewed the various treatment options for the condition.
Case Reports
Case 1
A 3-day-old male child was referred from the neonatal intensive care unit (ICU) presented for fever and rash since 2 nd day of birth. The child was born of a normal vaginal delivery with a normal Apgar score. On physical examination vitals were stable. Dusky reticulate rash surrounded by an erythematous halo over trunk and upper thighs [Figure - 1], blackish stellate patch over gluteal area and multiple hemorrhagic blebs [Figure - 2] were present. Systemic examination was found to be normal.
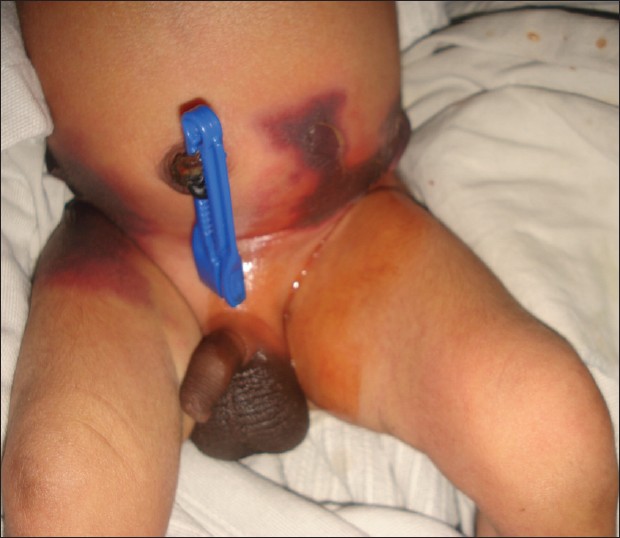 |
| Figure 1: Purpuric lesions on bilateral flanks in a 3-day-old neonate surrounded by an erythematous halo |
 |
| Figure 2: Close up view of the lesions on the right flank showing hemorrhagic blebs on a purpuric background surrounded by an erythematous halo |
On investigation, erythrocyte sedimentation rate was 01 mm/h, haemoglobin 14.3 g%, total leukocyte count 15,500/mm 3 with 80% neutrophils. Platelet count was 69,000/mm 3 . Prothrombin time (PT) was 19.6 s and INR 1.6. Protein C levels were 10% (reference 70-150). Protein S and antithrombin III levels were normal. Fibrinogen and fibrin degraded products (FDPs) levels were normal. Blood culture and culture from lesions was negative. Other parameters including serum electrolytes, kidney function tests, liver function tests, and 2D Echo were normal.
With a provisional diagnosis of neonatal purpura fulminans due to protein C deficiency, fresh frozen plasma transfusion was started along with empirical antibiotic therapy. The neonate was kept in the ICU for three days during which the patient was stable following which the parents refused treatment due to their inability to manage the rising expenses. On following up, we got to know that the neonate expired on the 5 th day of discharge from the hospital.
Case 2
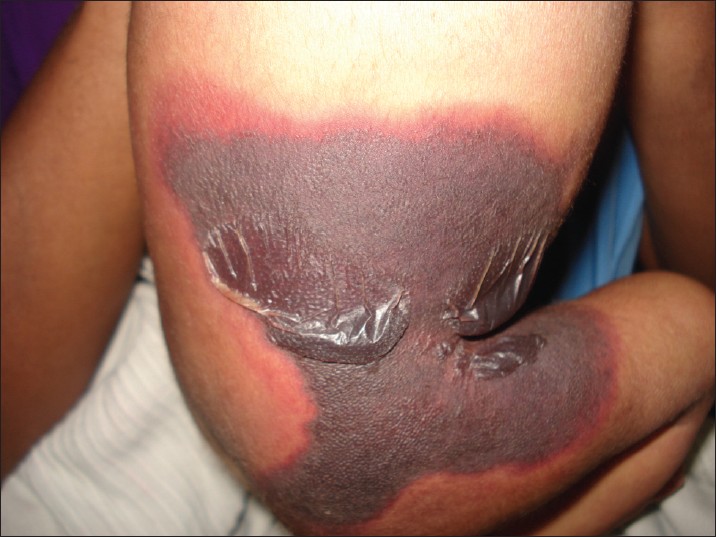
A 30-year-old male patient presented to us with high-grade fever and multiple purpuric rashes over the trunk and limbs. The skin rashes began on the 5 th day of fever and progressed to extensive areas of skin necrosis on the extensors of upper limbs, lower limbs, and ears. Necrotic areas were also present over the scrotum [Figure - 3] with gangrene of fingers and toes. The patient was drowsy and irritable on admission. His blood pressure was 70/50 and heart rate was 139 bpm. A provisional diagnosis of meningococcal septicaemia was considered.
 |
| Figure 3: Purpuric lesions on anterior thighs of 30-year-old male with necrosis of the scrotum exposing the testis |
On investigations, his haemoglobin was 12.3 g%, total leukocyte count was 30,000/mm 3 with 85% polymorphs. Platelet count was 42,000/mm 3 , INR 2.5, APTT ratio 2.18. Protein C levels were 42%. Protein S, and antithrombin III levels were normal. Culture from lesions was negative. FDP levels were significantly elevated (titres ++). Other parameters were blood urea 68 mg/dl; serum creatinine 4.2 mg/dl; total bilirubin 4.8 mg/dl; direct bilirubin 2.1 mg/dl; AST 284 U/l; ALT 172 U/l. Urine analysis revealed microscopic hematuria. A blood culture for Neisseria, however, proved to be negative and Weil Felix test was also negative. Serological tests (IgM antibody) for leptospirosis were positive and urine culture also showed positivity for leptospira. A final diagnosis of acute infectious purpura fulminans secondary to systemic leptospirosis was made in the patient.
The patient was treated initially with ceftriaxone and ionotropic agents. Multiple units of platelets and fresh frozen plasma were also infused. Later oral doxycycline was also added. The patient showed improvement within the second day of treatment. Fever subsided by the 5 th day of treatment and purpuric lesions gradually exfoliated. Minimal surgical debridement was done for the gangrenous lesions and amputation was performed for one finger later. On following up, the patient was doing well 1-month post-treatment.
Case 3
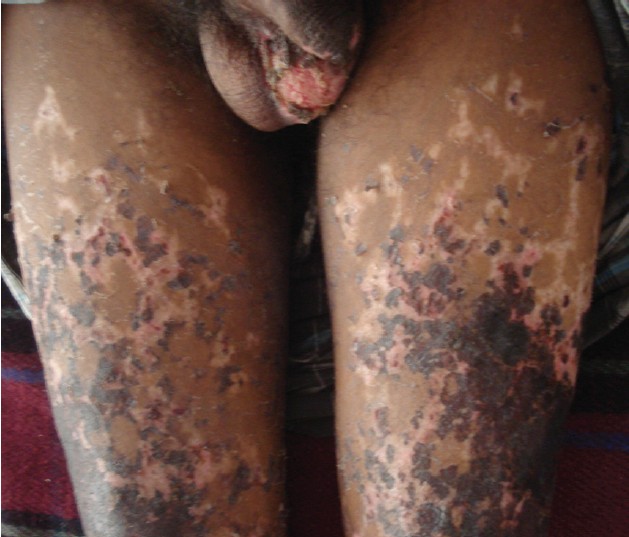
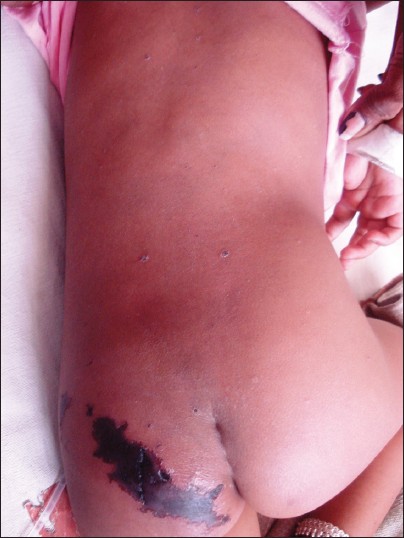
A 10-year-old girl presented to us with fever and multiple umbilicated vesicular lesions with crusting in various stages of evolution [Figure - 4]. On examination she also had a large purpuric patch on the left gluteal area 8 cm by 6 cm in size with retiform configuration [Figure - 5]. There was erythema surrounding the lesions and the margins were indurated and tender. Systemic examination was unremarkable. Vital parameters were within normal limits. On investigation, the various parameters were haemoglobin 10.8 g%, total leukocyte count 8000/mm 3 , platelet count 1,58,000/mm 3 . PT and INR were within normal range. Protein C levels were 68%. Protein S and antithrombin III levels were normal. FDP levels were normal. Blood culture and culture from lesions was negative. A diagnosis of idiopathic purpura fulminans following varicella zoster infection was made in the patient. The patient was transfused two units of fresh frozen plasma and empirical antibiotics were started. Later surgical debridement was done for the necrotic area. The patient was doing well post two months of surgery with almost complete healing of the necrotic area.
 |
| Figure 4: A 10-year-old girl with umbilicated vesicular lesions of varicella zoster on the back and well defined retiform purpuric lesions on the left gluteal area |
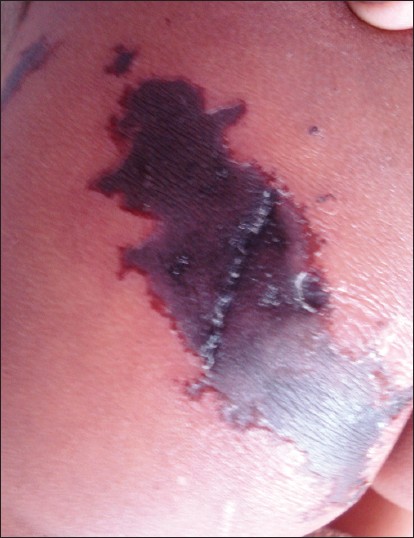 |
| Figure 5: Closer view of the purpuric lesion showing central dusky purpura and well defined, indurated border surrounded by an erythematous halo |
Discussion
Purpura fulminans is a life-threatening disorder characterized by sudden progressive cutaneous hemorrhage and necrosis. [3] The three forms of this disease are classified by the triggering mechanisms.
- Neonatal purpura fulminans.
- Idiopathic purpura fulminans.
- Acute infectious purpura fulminans.
Neonatal purpura fulminans generally presents within the first 72 h after birth, with purpuric lesions mainly over perineal region, flexor of thighs, and abdomen. Protein C mutations, inherited deficiency of protein S or antithrombin III may lead to neonatal PF.
Idiopathic purpura fulminans follows a bacterial or viral illness [4] and usually begins 7-10 days after the onset of the infection. Most cases occur in children. Varicella and streptococcal infections are most common. The pathogenesis involves acute transient decreases in protein C, protein S, or antithrombin III levels.
The most common form, acute infectious purpura fulminans, occurs superimposed on a bacterial infection. In this illness, the balance of anticoagulant and procoagulant endothelial cell activity is disturbed. This disturbance is precipitated by bacterial endotoxin which consumes antithrombin III as well as proteins C and S. [5] The two most common causes are meningococcus and varicella. Gram-negative bacilli and staphylococci have also been reported. We describe a case of acute infectious purpura fulminans secondary to systemic leptospirosis which to our best knowledge is the first reported case in world literature.
Lesions of PF are similar regardless of the precipitating condition. Its cardinal manifestations are presence of circumscribed ecchymosis of skin and symmetrical gangrene of the extremities with coagulation abnormalities suggestive of disseminated intravascular coagulation. [6] Primary lesions are non blanchable tender areas of purpura surrounded by an erythematous halo with indurated border. [7] Late findings are formation of bullae, which mark the development of hemorrhagic necrosis, and finally firm eschar, which ultimately sloughs. The distal extremities are often most severely involved. [3]
Typical hematological findings include low concentrations of fibrinogen, clotting factors and platelets, and prolonged prothrombin and partial thromboplastin times. [8] Fibrinogen degradation products tend to be raised and concentrations of proteins C, S, and antithrombin III reduced. [9] Fever and leucocytosis with a left shift are common.
Management must be tailored to the individual and involve supportive therapy and replacement of blood products and clotting factors. In acute infectious purpura fulminans, aggressive resuscitation, antibiotics and volume expansion are important. Correction of acid-base and electrolyte abnormalities is helpful. [9] Prompt excision of necrotic tissue is recommended and escharotomies may be indicated. [10] Many therapies have been used to arrest the progression of disease. Heparin bonds with antithrombin III to inhibit thrombus formation and may reverse the development of skin necrosis. [8] Protein C has got anticoagulant and anti-inflammatory properties, which contribute to improved survival. [11] In homozygous protein C deficiency, fresh frozen plasma (8-12 ml kg -1 ) can give effective replacement therapy. Antithrombin III replacement has been shown to normalize levels and reverse disseminated intravascular coagulation. [11] Recombinant tissue plasminogen activator (rtPA) induces fibrinolysis and improves peripheral perfusion in doses of 0.25-0.5 mg per kg per h. [12] Intravenous epoprostenol is a powerful vasodilator and has been used at doses of 5-20 ng per kg per min. [13] Plasmapheresis removes circulating endotoxin and assists in control of fluid balance. Fresh frozen plasma and cryoprecipitate as replacement fluids increase fibrinogen concentrations. Other therapies used include topical nitroglycerine, [14] i.v. dextran, [8] and leech saliva. [15]
| 1. |
Adcock DM, Bronza JP, Marlar RA. Proposed classification and pathologic mechanism of purpura fulminans and skin necrosis. Semin Thromb Hemost 1990;16:333-40.
[Google Scholar]
|
| 2. |
Urbaniak JR, O'Neil MT, Meyer LC. purpura fulminans. J Bone Joint Surg Am 1973;55:69-77.
[Google Scholar]
|
| 3. |
Darmstadt GL. Acute infectious purpura fulminans: Pathogenesis and medical management. Pediatr Dermatol 1998;15:169-83.
[Google Scholar]
|
| 4. |
Brown DL, Greenhalgh DG, Warden GD. purpura fulminans and transient protein C and S deficiency. Arch Dermatol 1998;124:119-23.
[Google Scholar]
|
| 5. |
Madden RM, Gill JC, Marlar RA. Protein C and protein S levels in two patients with acquired purpura fulminans. Br J Haematol 1990;75:112-7.
[Google Scholar]
|
| 6. |
Hautekeete ML, Berneman ZN, Bieger R, Stevens WJ, Bridts C, Buyssens N, et al. purpura fulminans in pneumococcal sepsis. Arch Intern Med 1986;146:497-9.
[Google Scholar]
|
| 7. |
Francis RB. Acquired purpura fulminans. Semin Thromb Haemostat 1990;16:310-25.
[Google Scholar]
|
| 8. |
Nolan J, Sinclair R. Review of management of purpura fulminans and two case reports. Br J Anaesth 2001;86:581-6.
[Google Scholar]
|
| 9. |
Smith OP, White B. Infectious purpura fulminans: Diagnosis and treatment. Br J Haematol 1999;104:202-7.
[Google Scholar]
|
| 10. |
Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J 1994;13:734-7.
[Google Scholar]
|
| 11. |
Cobcroft R, Henderson A, Solano C, Scott D. Meningococcal purpura fulminans treated with antithrombin III concentrate: What is the optimal replacement therapy? Aust N Z J Med 1994;24:575-6.
[Google Scholar]
|
| 12. |
Zenz W, Bodo Z, Zobel G. Recombinant tissue plasminogen activator restores perfusion in meningococcal purpura fulminans. Crit Care Med 1998;26:969-71.
[Google Scholar]
|
| 13. |
Stewart FJ, McClure BG, Mayne E. Successful treatment of neonatal purpura fulminans with epoprostenol. J R Soc Med 1991;84:623-4.
[Google Scholar]
|
| 14. |
Irazuzta J, McManus ML. Use of topically applied nitroglycerin in the treatment of purpura fulminans. J Pediatr 1990;117:993-5.
[Google Scholar]
|
| 15. |
de Chalain T, Cohen SR, Burstein FD. Successful use of leeches in the treatment of purpura fulminans. Ann Plast Surg 1995;35:300-4.
[Google Scholar]
|
Fulltext Views
10,365
PDF downloads
2,538





