Translate this page into:
Spontaneous regression of a congenital melanocytic nevus
2 Department of Pathology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, India
Correspondence Address:
Devinder Mohan Thappa
Department of Dermatology and STD, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry 605 006
India
| How to cite this article: Nath AK, Thappa DM, Rajesh NG. Spontaneous regression of a congenital melanocytic nevus. Indian J Dermatol Venereol Leprol 2011;77:507-510 |
Abstract
Congenital melanocytic nevus (CMN) may rarely regress which may also be associated with a halo or vitiligo. We describe a 10-year-old girl who presented with CMN on the left leg since birth, which recently started to regress spontaneously with associated depigmentation in the lesion and at a distant site. Dermoscopy performed at different sites of the regressing lesion demonstrated loss of epidermal pigments first followed by loss of dermal pigments. Histopathology and Masson-Fontana stain demonstrated lymphocytic infiltration and loss of pigment production in the regressing area. Immunohistochemistry staining (S100 and HMB-45), however, showed that nevus cells were present in the regressing areas.Introduction
Spontaneous regression rarely occurs in pigmented lesions such as melanocytic nevi as well as melanomas, and this process may be associated with the development of a depigmented halo or vitiligo. [1] There are, however, few reports of melanocytic nevi, including some giant nevi, which have involuted without an associated halo or vitiligo. [1],[2] Ulceration in congenital melanocytic nevus (CMN) in the neonatal period has also been reported to be associated with almost complete pigmentary regression in early childhood. [2] We hereby report a rare case of spontaneous regression in a CMN which was also associated with depigmentation in the lesion, and discuss the histopathology and immunohistochemistry study of biopsy specimens taken from different sites of the regressing lesion.
Case Report
A 10-year-old girl presented with hyperpigmented, raised skin lesion on the left leg since birth. There was no other cutaneous or systemic abnormality in the patient. She noticed whitish discoloration of the skin around the hyperpigmented lesion 6 months ago, which was associated with itching within the lower portion of the hyperpigmented lesion. Three months later she noted that the lower portion of the hyperpigmented lesion became flat and this was associated with progressive lightening of the color of the hyperpigmented lesion. A similar change subsequently occurred in the upper portion of the lesion as well. After 3 months, she also noticed a depigmented macule over the back.
On examination, a 7 Χ 5 cm 2 sized hyperpigmented, verrucous raised plaque was noted on the left leg [Figure - 1]. The upper peripheral portion of the plaque was black with terminal hairs. The upper central portion of the plaque was bluish without any hair. The lower portion was flat, almost flushed with the surrounding skin and showed whitish color with occasional small bluish spots, but no hair. Multiple hypopigmented and depigmented macules were seen around the upper, lower, and posterior aspects of the plaque, some of which were extending into the plaque especially in the lower portion. One 1.5 Χ 1 cm 2 sized depigmented macule was seen on the back. A diagnosis of spontaneously resolving CMN with acquired leukoderma and distant vitiligo-like depigmentation was made.
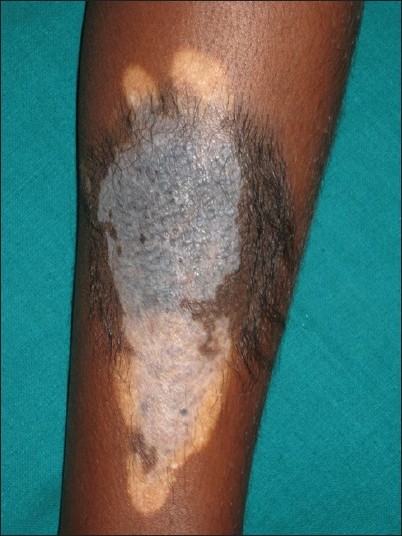 |
| Figure 1: Clinical image of CMN with the upper peripheral portion of the plaque showing black hyperpigmentation with terminal hairs, the upper central bluish hyperpigmented portion without any hair, the flat lower portion with spotty bluish pigmentation and peripheral leukoderma |
Dermoscopy of the CMN in the area of black hyperpigmentation showed typical thin and thick pigment network with coarse terminal hairs. In the intermediate zone (upper central portion of the plaque which was bluish hyperpigmented without any hair) cerebriform appearance, diffuse bluish veil, and complete absence of hair were noted [Figure - 2]. The lower flat portion of the plaque showed irregularly scattered islands of bluish spots and complete absence of hairs.
Histopathological examination from the upper hyperpigmented portion (bearing terminal hairs) of the plaque showed nests and cords of nevus cells with melanin pigmentation in papillary and reticular dermis circumferentially surrounding the adnexal structures confirming the diagnosis of CMN [Figure - 3]. Masson-Fontana stain was strongly positive in the nevus cells as well as in epidermal melanocytes [Figure - 4]. Immunohistochemistry showed positive staining for S100 and HMB-45 in nevus cells. Histopathological examination from the lower portion (area of flattening and depigmentation) showed dense infiltration by sheets of lymphocytes around the nevus cells in superficial dermis and adnexal structures. The lymphocytic infiltrate obscured the nevus cells in most of the sections studied [Figure - 4]. Melanin pigmentation was not evident on hematoxylin and eosin staining. There was elastotic degeneration of collagen in the dermis. Immunohistochemistry for S100 and HMB-45 showed positive staining in nevus cells [Figure - 5], but Masson-Fontana stain for melanin was negative in nevus cells.
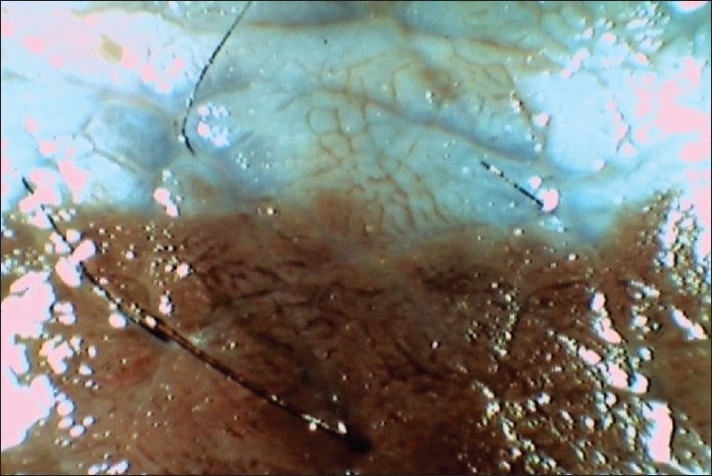 |
| Figure 2: Dermoscopy image of the margin between persisting CMN and recent regression showing cerebriform appearance and diffuse bluish veil in the area of regression (signifying loss of epidermal pigmentation) |
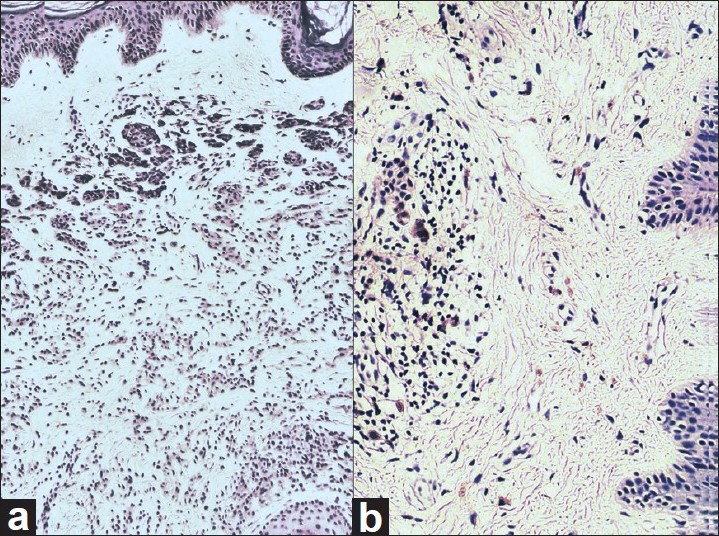 |
| Figure 3: (a) Histopathology section from hyperpigmented region of CMN with nests of nevus cells extending deep into reticular dermis surrounding a hair follicle (H and E, ×100). (b) Histopathology section from the lower portion of the regressing plaque shows sheets of lymphocytes in dermis surrounding the occasional nevus cells (H and E, ×100) |
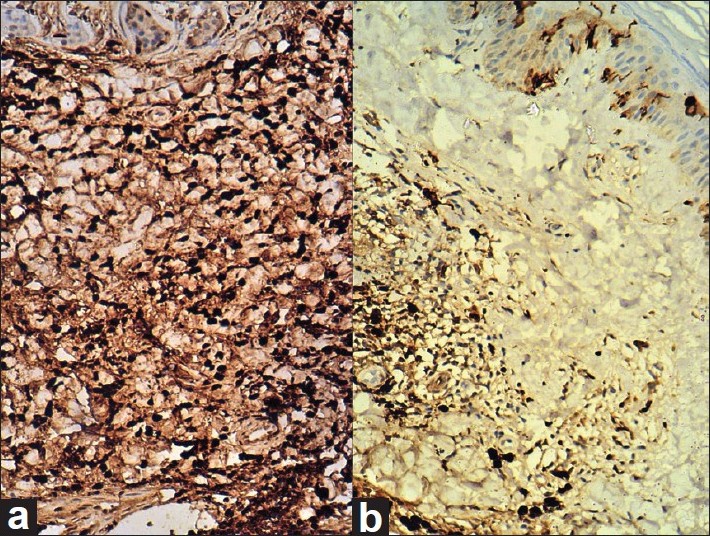 |
| Figure 4: (a) Histopathology section from the hyperpigmented region of CMN showing strong positivity for melanin pigmentation in basal layer melanocytes (arrow head) and in nevus cells (arrow) (Masson-Fontana Stain, ×100). (b) Histopathology section from the lower portion of the regressing plaque shows the absence of melanin pigmentation in nevus cells as well as in basal layer melanocytes in epidermis (Masson-Fontana stain, ×100) |
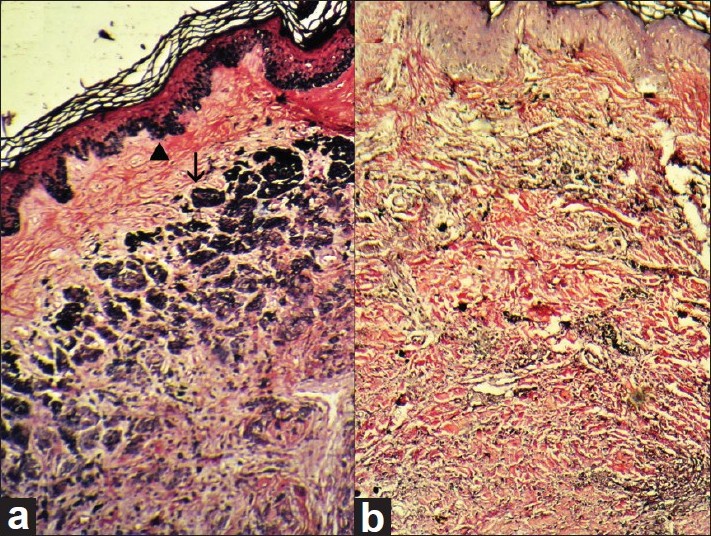 |
| Figure 5: Immunohistochemistry with S100 antibody (polymer peroxidase system DAB chromogen, ×100) showing positivity in nevus cells from (a) hyperpigmented region of CMN. (b) The lower portion of the regressing plaque |
Discussion
Halo nevus, also called Sutton′s nevus [3] or leukoderma acquisitum centrifugum, [4] defines development of depigmented zones around dermal, junctional, compound nevus, Spitz nevus, and blue nevus. [5] Halo phenomenon may also develop around Mongolian spot, cafι au lait macules, neurofibroma, basal cell carcinoma, seborrheic keratosis, histiocytoma, and flat warts. [3] The clinical evolution is characterized by progressive lightening followed by disappearance of the central nevus with persistence of leukoderma for many years. [3] It is seen in 1% of Caucasians. [5] Halo nevi usually occur during childhood or adolescence. [3] The depigmentation may occur within the lesion, around it or at a distant site. [5] It is mostly sporadic; however, familial clustering has been reported. [5] Halo phenomenon may develop around melanoma spontaneously or following IL-2/IFN therapy and it signifies a better prognosis. [5]
The mechanism of halo phenomenon is supposed to involve T-cell immunity and the presence of IgM autoantibodies. [4],[5] About 80% of mononuclear infiltrates in halo nevus consists of T lymphocytes with high suppressor/cytotoxic T cells. [4] The nevomelanocytes express MHC class I antigens allowing them to be recognized as target cells for cytotoxic T cells. [4] The nevus cells in the halo nevus may be monoclones of aberrant cells, and the halo phenomenon may represent vigorous host response against these aberrant clones. [6] The occasional association of halo nevus with melanoma has led to the hypothesis that cytotoxic T cells acting against melanoma cells also attack nevocellular cells sharing common antigenic determinants with melanoma cells. [4] Halo phenomenon may be due to loss of melanin synthesis and/or apoptosis of melanocytes. [1],[2] Loss of melanin synthesis may be a more plausible explanation as the number of dermal nevus cells may not reduce much, they rather become amelanotic. [2] The induction of host immune response could actually become a therapeutic option in congenital melanocytic nevi. [6]
Spontaneously resolving CMN with leukoderma and vitiligo-like depigmentation at a distant site as was noted in our case was a rare occurrence. Itin et al. [5] described a 6-year-old boy who had progressive depigmentation of CMN which was later on followed by the development of whitish ring around the CMN and depigmented lesions at distant sites after 1 year. Langer and Konrad [4] described a 7-year-old boy who had progressive halo of depigmentation around CMN with vitiligo lesions at distant sites. According to Sutton, [3] the halo should be uniform and concentric completely surrounding the melanocytic lesion reproducing its shape. However, the classical uniformly concentric halo formation was not seen in our case. Long-term prognosis of halo congenital nevi, their natural course, and risk of malignant melanoma occurring in them have not been adequately studied in large series. However, anecdotal reports of spontaneous resolution of halo CMNs as well as development of malignant melanoma in them exist in the literature. [7]
Dermoscopy of the hyperpigmented portion of the nevus was consistent with the typical patterns seen in CMN. The areas of recent regression (upper central portion of the plaque which was bluish hyperpigmented without any hair) showed loss of hair, cerebriform appearance, and bluish veil which was due to persistent dermal pigments in an area where the overlying epidermal pigment was lost. However, only scattered bluish spots were seen in the lower flat portion of the plaque, whereas there was no bluish spot at all in areas of complete depigmentation signifying progressive loss of the dermal pigments with regression.
In histopathology, CMN may be junctional, compound, or intradermal nevus, and its location in the dermis may be either superficial (which may include junctional involvement) or superficial and deep. [8] CMN may have the similar histologic appearance as acquired nevi, but differ from acquired nevi by (a) the presence of melanocytes in and around hair follicles, in sweat ducts and glands, in sebaceous glands, in or in intimate association with vessel walls, in arrector pili muscles, and in the perineurium of nerves, (b) extension of melanocytes between collagen bundles singly or in double rows, and (c) extension into the deepest reticular dermis and the subcutis. [8] The cells of CMN are typically positive for the melanocytic markers S100, HMB-45, and Melan-A. [8] Hogan et al. [6] noted modest lymphocytic infiltrate in the area of clearance of the nevus. Langer and Konrad [4] reported two cases of CMN with halo phenomenon where a dense, band-like upper dermal mononuclear cells infiltrate was noted throughout the nevus, but not in the halo. In our case, the area of regression and depigmentation showed lymphocytic infiltrate. By Masson-Fontana stain and immunohistochemistry, we demonstrated that the nevus cells persisted without any tendency towards pigment production in the area of regression.
We conclude that spontaneous regression of a CMN starts with loss of terminal hairs and epidermal pigments resulting in bluish color of the plaque, which is followed by flattening and loss of dermal pigments resulting in vitiligo-like leukoderma. Dermal nevus cells, however, do not disappear, they just lose the ability to produce melanin pigments. The process of regression is associated with lymphocytic infiltration.
| 1. |
Martín JM, Jordá E, Calduch L, Alonso V, Revert A. Progressive depigmentation of a palmar congenital melanocytic nevus without an associated halo phenomenon. Dermatology 2006;212:198-9.
[Google Scholar]
|
| 2. |
Gass JK, Grant JW, Hall PN, Atherton DJ, Burrows NP. Clinical resolution of a neonatally eroded giant congenital melanocytic nevus. Pediatr Dermatol 2006;23:567-70.
[Google Scholar]
|
| 3. |
Arpaia N, Cassano N, Filotico R, Laricchia F, Vena GA. Unusual clinical presentation of regression in a congenital melanocytic nevus. Dermatol Surg 2005;31:471-3.
[Google Scholar]
|
| 4. |
Langer K, Konrad K. Congenital melanocytic nevi with halo phenomenon: Report of two cases and a review of the literature. J Dermatol Surg Oncol 1990;16:377-80.
[Google Scholar]
|
| 5. |
Itin PH, Lautenschlager S. Acquired leukoderma in congenital pigmented nevus associated with vitiligo-like depigmentation. Pediatr Dermatol 2002;19:73-5.
[Google Scholar]
|
| 6. |
Hogan DJ, Murphy F, Bremner RM. Spontaneous resolution of a giant congenital melanocytic nevus. Pediatr Dermatol 1988;5:170-2.
[Google Scholar]
|
| 7. |
Pearl H, Jaber R, Mehregan D. Acquired leukoderma in a regressing congenital nevomelanocytic nevus. Int J Dermatol 2009;48:870-2.
[Google Scholar]
|
| 8. |
Elder DE, Elenitsas R, Murphy GF, Xu X. Benign pigmented lesions and malignant melanoma. In: Elder DE, Elenitsas R, Johnson BL, Murphy GF, editors. Lever's Histopathology of the Skin. 9 th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. p. 742-5.
th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. p. 742-5.'>[Google Scholar]
|
Fulltext Views
6,451
PDF downloads
2,694





