Translate this page into:
Sporotrichoid nocardiosis with cutaneous dissemination
Correspondence Address:
Sanjay S Bosamiya
Department of Dermatology, Surat Municipal Institute of Medical Education & Research (SMIMER), Umarwada, Surat - 395 009, Gujarat
India
| How to cite this article: Bosamiya SS, Vaishnani JB, Momin AM. Sporotrichoid nocardiosis with cutaneous dissemination. Indian J Dermatol Venereol Leprol 2011;77:535 |
Abstract
Dissemination of primary cutaneous nocardiosis is a rare event. A 37-year-old man working as farmer presented with multiple painful suppurative nodular and ulcerative skin lesions over left lower extremities, in a linear pattern, with duration of five months and single painful nodule over right elbow since last three months. We found the presence of beaded filamentous bacteria in Gram stain smear and partial acid fast stain, from the smear taken from pus. Patient responded well to cotrimoxazole therapy. Hence, we confirm our diagnosis of sporotrichoid pattern of cutaneous nocardiosis with dissemination to other cutaneous area.Introduction
Nocardiosis in human beings was often considered rare, but now-a-days, it is being increasingly encountered in clinical practice. [1] The clinical manifestations and the severity of the disease and the prognosis in an infected patient are extremely variable. [2] These are mostly determined by certain factors such as the route of infection and the immune status of the patient. Although the lungs are the primary organs affected by the nocardia species, seen in immunocompromised individual, cutaneous nocardiosis may also occur as a primary disease, seen in an immunocompetent individual. [3] Primary cutaneous nocardiosis with dissemination is relatively rare. [4] Here, we report a rare, sporotrichoid pattern of cutaneous nocardiosis with dissemination to other cutaneous area.
Case Report
A 37-year-old man working as farmer presented with multiple painful suppurative nodular and ulcerative skin lesions over left lower extremities in a linear pattern, with duration of five months and single painful nodule over right elbow since last three months. Patient was not able to recollect any history of trauma on foot.
Lesions had first started on planter surface of left foot as painful nodules, gradually spread to involve dorsa of left foot, left leg, and left thigh in a linear pattern. Nodules had ruptured with discharge of pus and ulcer formation. Since last three months, patient had developed a painful nodule over right elbow.
Cutaneous examination revealed edema of left foot, with multiple pus discharging sinuses over medial aspect, with surrounding hyperpigmented skin [Figure - 1]. Multiple ulcers with slough were present on medial aspect left leg and thigh with thick cord-like structure palpable in between the ulcers with surrounding hyperpigmented skin, forming a linear pattern of distribution [Figure - 2]. Lymph node examination revealed enlargement of left femoral lymph nodes. Tender nodule was present on right elbow with overlying hyperpigmented skin [Figure - 3].
 |
| Figure 1: Edema of left foot with discharging sinuses and hyperpigmented skin |
 |
| Figure 2: Multiple ulcers with slough present on medial aspect left leg and thigh in linear sporotrichoid pattern |
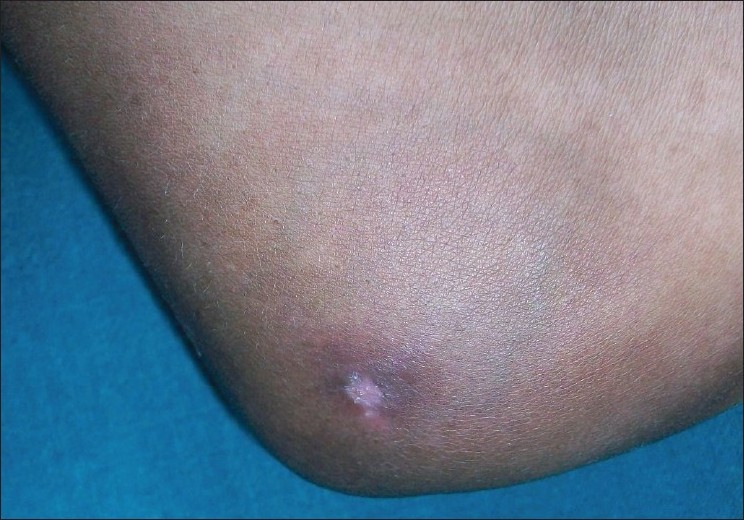 |
| Figure 3: Hyperpigmented nodule on right elbow |
With clinical differential diagnosis of sporotrichosis, eumycetoma, actinomycetoma, fish tank granuloma, infection with other atypical mycobacteria, nocardiosis, and osteomyelitis, we had collected pus for potassium hydroxide (KOH) smear, Gram stain, and acid fast stain. We found presence of beaded filamentous bacteria in Gram stain [Figure - 4], while rest of the smears had no positive findings. With this consideration, we again collected pus for partial acid fast stain (used 1% concentration of sulphuric acid), with again same positive finding of presence of filamentous bacteria in smear [Figure - 5]. Culture was done on Sabouraud Dextrose agar and Lowenstein-Jenson′s medium, but unfortunately, there was no growth after seven and 21 day of incubation. We had taken the biopsy from ulcer on medial aspect of left lower leg. Histopathological examination with HandE stain revealed granulomatous reaction with collection of neutrophils, lymphocytes, and scattered giant cells [Figure - 6].
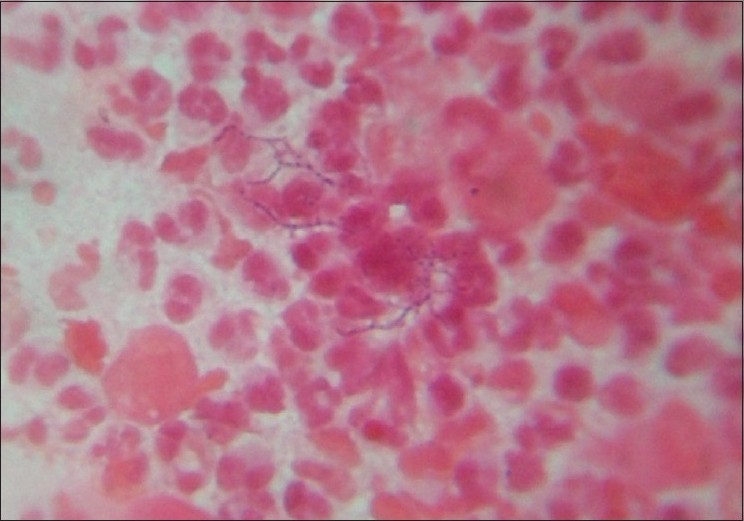 |
| Figure 4: Presence of beaded filamentous bacteria on gram stain |
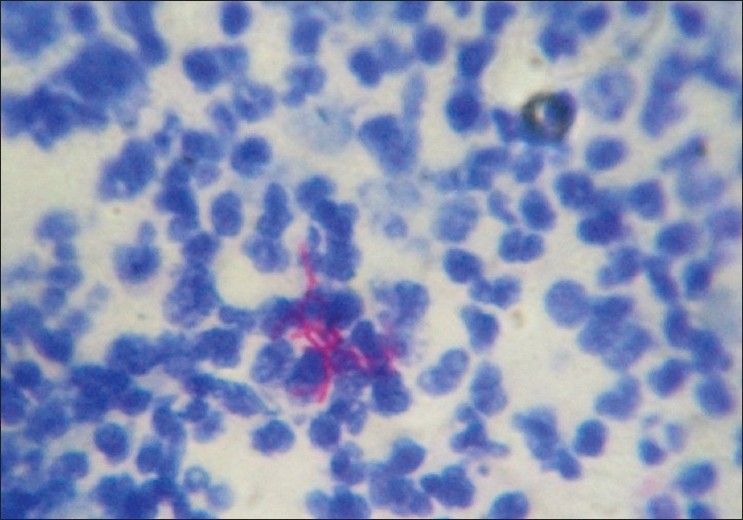 |
| Figure 5: Presence of beaded filamentous bacteria on modified acid fast stain |
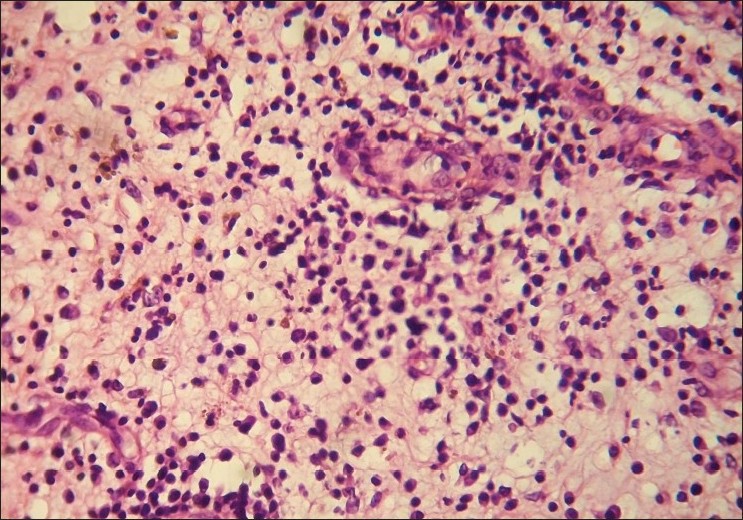 |
| Figure 6: Dermal inflammatory infiltrates of neutrophils and lymphocytes (H and E, ×400) |
Other laboratory investigations revealed hemoglobin of 8.0 gm %; total white blood cell count, 17 700/cumm; and differential count, P-93%, L-05%, M-01%, E-01%, B-00% with erythrocyte sedimentation rate (ESR) of 15 mm/h. Liver and renal function tests were normal. Random blood sugar (RBS) was 93 mg %. Serology for human immunodeficiency virus (HIV), hepatitis C virus (HCV), and Venereal Disease Research Laboratory (VDRL) test was nonreactive and for HbsAg was found to be positive. X-ray chest was normal with negative Mantoux test. No abnormalities were detected in x-ray of left foot, leg and thigh, right elbow except soft tissue swelling.
With above clinical finding, positive microscopy, and granulomatous reaction on biopsy, we confirmed our diagnosis of sporotrichoid pattern of cutaneous nocardiosis with dissemination to distant cutaneous site; and started treatment with cotrimoxazole therapy in dose of trimethoprim 160 mg and sulphamethoxazole 800 mg twice daily. Excellent results were observed within seven days of therapy [Figure - 7], including the lesion over the elbow.
 |
| Figure 7: Excellent response with cotrimoxazole therapy |
Discussion
Cutaneous nocardiosis is acute-to-chronic suppurative disease caused by genus Nocardia, an aerobic actinomycetaceae. Nocardia species are thin, aerobic, Gram-positive, weakly acid-fast bacilli that form beaded branching filaments and produce fungus-like colonies in culture. These are saprophytic bacteria which are found worldwide in soil, decaying vegetable material, and water. The first description of Nocardia came from a French veterinarian, Edmond Nocard, in 1888, in relation to bovine farcy. [5] The exact incidence of primary cutaneous nocardiosis is not clear. The number of cases of primary cutaneous nocardiosis is very few. According to Palmer et al., [6] the incidence of primary cutaneous nocardiosis reported in the English literature between 1961 and 1971 was 5%. Though data regarding the overall incidence of primary cutaneous Nocardiosis are not available from India, a few cases have been documented. [7]
The mode of transmission is accidental inoculation. It is prevalent among the rural population where agriculture is the main way of livelihood. Adult males, especially bare-foot walkers, are the common sufferers.
Cutaneous nocardiosis presents either as a part of disseminated infection or as a primary infection resulting from inoculation. The following three clinical variants of primary cutaneous nocardiosis have been identified: (1) Mycetoma; (2) Superficial skin infections such as cellulitis, abscesses, ulcers, or granulomas; and (3) Lymphocutaneous (sporotrichoid). Mycetoma is the commonest clinical pattern of primary cutaneous nocardiosis. [7]
The lymphocutaneous pattern of the disease is the rarest type and commonly occurs in otherwise healthy individuals. [3] Only seven such cases are published in Indian literature [Table - 1]. Nocardia brasiliensis is the most frequently isolated organism form lymphocutaneous variety. Since this form of the disease bears a striking clinical resemblance to a superficial infection with the dimorphic fungus Sporothrix schenckii, it has also been termed the sporotrichoid form of cutaneous nocardiosis. Clinically, it presents with painful suppurative nodules at the site of inoculation followed by discharge of pus and ulcer formation. Usually, there is no history of discharge of granules. As the disease progress, new lesions (abscesses) develop along the line of lymphatics, leading to clinical pattern of nodular lymphangitis. And, when regional lymph node involvement occurs, this form of the disease is referred to as the lymphocutaneous syndrome.
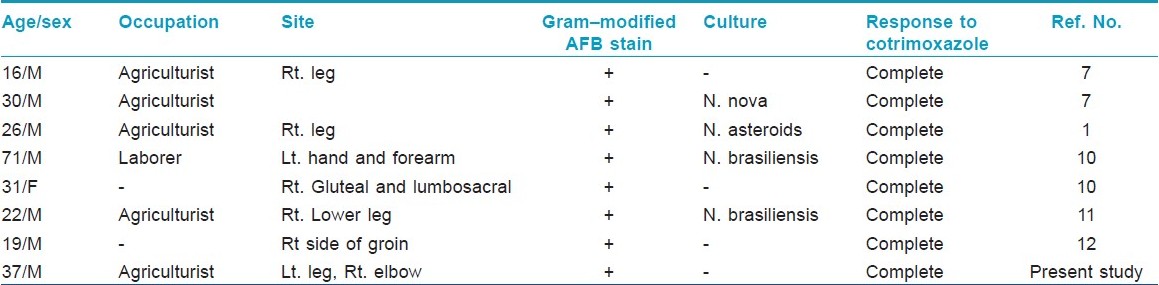
Dissemination of primary cutaneous nocardiosis is very rare, is often a late event, and is most frequently seen in pulmonary nocardiosis. [4] However, dissemination may occur very rarely from primary cutaneous lesions to anatomic distant sites. In such cases, the central nervous system is the most common site of involvement resulting in cerebritis or abscess formation. [8] We report the case of sporotrichoid nocardiosis that disseminated from left lower leg to right elbow.
Main differential diagnosis includes sporotrichosis, infections by atypical mycobacteria (M. marinum and M. chelonae), and Leishmania braziliensis. Nocardiosis differ from above conditions by its acute onset, erythema of the overlying skin, tenderness, and a highly inflammatory course.
Demonstration of the organism from clinical specimens like pus or aspirated fluid from an unruptured nodule by Gram stain and modified acid fast bacilli stain is the mainstay of diagnosis. [7] Gram-positive and partial acid fast, thin, beaded, branching filaments are the characteristic appearance of the organism. Identification of the Nocardia species by culture is a tedious process.[12]
Cotrimoxazole is the therapy of choice for proven or presumed nocardiosis. Drug therapy should be continued for 6 to 18 months, depending on the extent of the disease, because of the high incidence of relapse and metastatic abscesses with shorter duration of therapy. [9] Other effective drugs are dapsone, amikacin, amoxycillin, cephalosporins, minocycline, erythromycin, ciprofloxacin, clindamycin, and imipenem. [5]
Unfortunately, we could not isolate the species of Nocardia causing infection in our patient. However, clinical findings, microscopic demonstration of typical morphology of the organism, histopathological evidence of granuloma, and dramatic therapeutic response to cotrimoxazole helped to confirm our clinical impression.
| 1. |
Baradkar VP, Mathur M, Kulkarni SD, Kumar S. Sporotrichoid pattern of cutaneous nocardiasis due to Nocardia asteroids. Indian J Pathol Microbiol 2008;51:432-4.
[Google Scholar]
|
| 2. |
Angelika J, Hans-Jürgen G, Uwe-Frithjof H. Primary Cutaneous Nocardiosis in a husband and wife. J Am Acad Dermatol 1999;41:338-40.
[Google Scholar]
|
| 3. |
Tsuboi R, Takamori K, Ogawa H, Mikami Y, Arai T. Lymphocutaneous nocardiosis caused by Nocardia asteroides. Arch Dermatol 1986;122:1183-5.
[Google Scholar]
|
| 4. |
Beaman BL, Beaman L. Nocardia species: Host-parasite relationship. Clin Microbiol Rev 1994;7:213-64.
[Google Scholar]
|
| 5. |
McNeil MM, Brown JM. The medically important aerobic actinomycetes: Epidemiology and microbiology. Clin Microbiol Rev 1994;7:357-417.
[Google Scholar]
|
| 6. |
Palmer DL, Harvey RL, Wheeler JK. Diagnostic and therapeutic considerations in Nocardia asteroides infection. Medicine 1974;53:391-401.
[Google Scholar]
|
| 7. |
Inamadar AC, Palit A. Primary cutaneous nocardiosis: A case study and review. Indian J Dermatol Venereol Leprol 2003;69:386-91.
[Google Scholar]
|
| 8. |
Lakshmi V, Sundaram C, Meena AK, Murthy JM. Primary cutaneous nocardiosis with epidural abscess caused by Nocardia brasiliensis: A case report. Neurol India 2002;50:90.
[Google Scholar]
|
| 9. |
Lerner PI. Nocardiosis. Clin Infect Dis 1996;22:891-905.
[Google Scholar]
|
| 10. |
Sharma NL, Mahajan VK, Agarwal S, Katoch VM, Das R, Kashyap M, et al. Nocardial mycetoma: Diverse clinical presentations. Indian J Dermatol Venereol Leprol 2008;74:635-40.
[Google Scholar]
|
| 11. |
Ramani R, Kumari G. Spontaneous remission of primary cutaneous nocardiosis. Indian J Dermatol Venereol Leprol 1993;59:37-8.
[Google Scholar]
|
| 12. |
Belliappa AD, Sukumar D, Shetty JN, Nandakishore B. Lymphocutaneous nocardiosis presenting as inguinal bubo. Indian J Dermatol 2003;48:164-6.
[Google Scholar]
|
Fulltext Views
3,672
PDF downloads
2,631





