Translate this page into:
Sulfasalazine and pentoxifylline, a new adjuvant in young pemphigus patients: A pilot study
Correspondence Address:
Devraj Dogra
Department of Dermatology, Venereology and Leprosy, Government Medical College and Associated Hospitals, Shalamar Road, Jammu - 180 001, Jammu and Kashmir
India
| How to cite this article: Dogra D, Dogra NK, Gupta N, Gupta V. Sulfasalazine and pentoxifylline, a new adjuvant in young pemphigus patients: A pilot study. Indian J Dermatol Venereol Leprol 2015;81:640-642 |
Sir,
Treatment of pemphigus with dexamethasone cyclophosphamide pulse therapy is effective, but the use of cyclophosphamide in young patients is relatively contraindicated due to the risk of gonadal dysfunction and infertility.[1] Alternative immunosuppressants to cyclophosphamide are azathioprine, mycophenolate mofetil, methotrexate and cyclosporine but these do not induce remission in all patients and are contraindicated in some.[1] Tumor necrosis factor-α (TNF-α) is a cytokine that contributes to acantholysis in pemphigus.[2] Drugs that act against TNF-α, such as sulfasalazine and pentoxifylline have been reported as an effective adjunctive treatment in pemphigus. They help by reducing the serum levels of TNF-α, resulting in rapid clinical improvement.[3] Sulfasalazine inhibits TNF-α messenger RNA levels and binds to circulating TNF-α whereas pentoxifylline reduces its production by inhibiting phosphodiesterase.[4],[5] These low cost alternatives are easily available. The present study was undertaken to evaluate the effectiveness of sulfasalazine and pentoxifylline as an adjuvant to dexamethasone pulse therapy instead of cyclophosphamide. It was used in unmarried pemphigus patients, those who wished to conceive and as an additional adjuvant in patients not responding to dexamethasone cyclophosphamide pulse therapy.
Fifteen patients suffering from pemphigus were enrolled in the study between January 2010 and January 2014. The diagnosis was based on clinical features, Tzanck smear and histopathology. The severity of disease was graded as mild, moderate or severe based on Kumar's scoring system.[6] Patients with uncontrolled diabetes, hypertension, severe systemic disease and pregnant or lactating women were excluded from the study. All of them were hospitalized and treated after taking informed consent. Relevant investigations included complete haemogram, urine analysis, stool for occult blood, renal and liver function tests, glucose-6-phosphate dehydrogenase (G6PD) levels, chest X-ray and electrocardiogram. All investigations were repeated at monthly intervals in phase I and II, except for chest X-ray and G6PD levels. The investigations were repeated every 3 months in phase III. Semen analysis was done in all male patients before initiating treatment, and repeated after completion of phase III. The modified dexamethasone pulse therapy comprised of four phases.
- Phase I: Dexamethasone 100 mg in 500 ml of 5% dextrose was given as a slow infusion over 2 hours for 3 consecutive days. Such pulses were repeated every 28 days until no new lesions appeared and all old ones healed. Along with this, oral sulfasalazine 500 mg and pentoxifylline 400 mg were given thrice daily. Oral corticosteroids and interval pulses were also given to reduce the duration of phase I
- Phase II: Consisted of dexamethasone pulse therapy with sulfasalazine and pentoxifylline given orally for a fixed duration of 9 months
- Phase III: Only oral sulfasalazine and pentoxifylline were given for 9 months
- Phase IV: All the drugs were withdrawn and the patients were followed up for as long as possible.
Among the fifteen patients enrolled in the study, 13 (86.7%) were new cases who were unmarried or wished to conceive and two (13.3%) were not responding to conventional dexamethasone cyclophosphamide therapy. Fourteen (93.3%) patients were diagnosed to have pemphigus vulgaris and one (6.67%) had pemphigus foliaceus. Eleven (73.3%) patients had severe disease with more than 30% body surface area involvement and/or extensive oral mucosal involvement. Four (26.7%) patients had moderate disease. The mean age of presentation was 25.3 years (ranging from 12 to 40 years) [Table - 1]. The age distribution of our study is consistent with the findings of Kanwar and De, who observed that the onset of pemphigus is more common in the 21-40 years age group.[1] The male to female ratio was 3:2 (nine males and six females). The duration of disease before treatment initiation varied from 2 months to 2½ years.

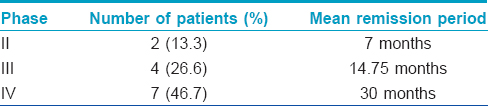
We lost two patients to follow-up and the remaining completed phase I with an average of five pulses. Two (13.3%) of these are in phase II, four (26.7%) in phase III and seven (46.7%) are in phase IV with remission periods ranging from 6 months to 3 years [Table - 2]. The remission rates and the number of pulses required to achieve remission are comparable to those on conventional dexamethasone cyclophosphamide pulse therapy.[7],[8] Kandan and Thappa reported that the majority of their patients needed 6–10 pulses to achieve remission in phase I. Pasricha et al.[7],[8] also observed similar findings where nearly 50% of patients achieved remission with six or fewer dexamethasone cyclophosphamide pulses. In the present study, only one patient relapsed after 2½ years, with mild oral lesions. Similar observations were made by Kandan and Thappa who reported remission in 87.5% of cases who had completed phase I of pulse therapy.[7] Pasricha et al. achieved remission in 84% of the patients treated with dexamethasone cyclophosphamide pulse therapy, and the relapse rate was 31%, mostly seen in those who defaulted during the treatment.[8]
One of our patients was not in remission even after completing two years of conventional dexamethasone cyclophosphamide pulse therapy. Adding sulfasalazine and pentoxifylline initiated complete healing of the persistent lesions and no new lesions have appeared after five months of therapy. This patient received nine regular pulses and five interval pulses in phase I and is now in phase IV since one year.
The side effects associated with prolonged use of corticosteroids, sulfasalazine and pentoxifylline were minimal. The common side effects seen were nausea, vomiting, headache and fatigue, which was seen in seven (46.7%) patients. Acneiform eruptions occurred in five (33.3%) patients, mild elevation in liver enzymes in two (13.3%) and plane warts over the face in one patient (6.67%). Oligospermia can occur in males who take sulfasalazine for a long period. The effects are found to be reversible in 2½ months after stopping the drug.[9] The sperm count and motility in the pre-treatment and post-treatment samples of the three male patients who had completed treatment were not significantly altered.
Although the number of patients in our study is small, and the facilities to measure desmoglein antibodies and TNF-α levels are not available, our results are comparable with the larger series of pemphigus patients who have been treated with dexamethasone cyclophosphamide pulse therapy.[7],[8] Sulfasalazine and pentoxifylline are inexpensive drugs, have relatively fewer side effects and are not immunosuppressive. We therefore recommend that cyclophosphamide may be replaced with these adjuvants, especially for younger patients. Further studies with a control group would help to establish the usefulness of sulfasalazine and pentoxifylline.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kanwar AJ, De D. Pemphigus in India. Indian J Dermatol Venereol Leprol 2011;77:439-49.
[Google Scholar]
|
| 2. |
Feliciani C, Toto P, Amerio P, Pour SM, Coscione G, Shivji G, et al. sIn vitro andin vivo expression of interleukin-1alpha and tumor necrosis factor-alpha mRNA in pemphigus vulgaris: Interleukin-1alpha and tumor necrosis factor-alpha are involved in acantholysis. J Invest Dermatol 2000;114:71-7.
[Google Scholar]
|
| 3. |
el-Darouti M, Marzouk S, Abdel Hay R, el-Tawdy A, Fawzy M, Leheta T, et al. The use of sulfasalazine and pentoxifylline (low-cost antitumour necrosis factor drugs) as adjuvant therapy for the treatment of pemphigus vulgaris: A comparative study. Br J Dermatol 2009;161:313-9.
[Google Scholar]
|
| 4. |
Bissonnette EY, Enciso JA, Befus AD. Inhibitory effects of sulfasalazine and its metabolites on histamine release and TNF-alpha production by mast cells. J Immunol 1996;156:218-23.
[Google Scholar]
|
| 5. |
Deree J, Martins JO, Melbostad H, Loomis WH, Coimbra R. Insights into the regulation of TNF-alpha production in human mononuclear cells: The effects of non-specific phosphodiesterase inhibition. Clinics (Sao Paulo) 2008;63:321-8.
[Google Scholar]
|
| 6. |
Kumar B, Arora S, Kumaran MS, Jain R, Dogra S. Study of desmoglein 1 and 3 antibody levels in relation to disease severity in Indian patients with pemphigus. Indian J Dermatol Venereol Leprol 2006;72:203-6.
[Google Scholar]
|
| 7. |
Kandan S, Thappa DM. Outcome of dexamethasone-cyclophosphamide pulse therapy in pemphigus: A case series. Indian J Dermatol Venereol Leprol 2009;75:373-8.
[Google Scholar]
|
| 8. |
Pasricha JS, Khaitan BK, Raman RS, Chandra M. Dexamethasone-cyclophosphamide pulse therapy for pemphigus. Int J Dermatol 1995;34:875-82.
[Google Scholar]
|
| 9. |
O'Moráin C, Smethurst P, Doré CJ, Levi AJ. Reversible male infertility due to sulphasalazine: Studies in man and rat. Gut 1984;25:1078-84.
[Google Scholar]
|
Fulltext Views
3,188
PDF downloads
2,223





