Translate this page into:
Symmetrical peripheral gangrene
Correspondence Address:
Sudip Kumar Ghosh
Department of Dermatology, Venereology, and Leprosy, R.G. Kar Medical College, 1, Khudiram Bose Sarani, Kolkata-700 004
India
| How to cite this article: Ghosh SK, Bandyopadhyay D. Symmetrical peripheral gangrene. Indian J Dermatol Venereol Leprol 2011;77:244-248 |
Introduction
Symmetrical peripheral gangrene (SPG) is a well-documented but rare clinical syndrome characterized by symmetrical distal ischemic damage leading to gangrene of two or more sites in the absence of large vessel obstruction or vasculitis. [1],[2],[3] The term ′purpura fulminans′, often used synonymously, does not adequately depict this specific scenario. [3] Rather, purpura fulminans is characterized by acute onset, rapidly progressive purpuric lesions leading to skin necrosis, gangrenous changes of limbs or digits and organ dysfunction. [4] Hutchison first described SPG in 1891. [5] Although described more than a century ago, most of the cases of SPG have been documented as single case reports. A few relatively large case series have been described recently. [3],[6],[7]
A wide array of infective and noninfective etiological factors has been linked with the development of SPG. The exact pathomechanism of the vascular occlusion in SPG is uncertain. A low-flow state alongwith disseminated intravascular coagulation (DIC) is usually present. [8],[9] SPG is a cause of significant morbidity and mortality often requiring multiple limb amputations in the survivors. Thus, early recognition of SPG and its underlying conditions can have profound impact on the management of the condition and its final outcome.
Etiopathogenesis
The pathogenesis of SPG is not well understood. A low-flow state is commonly present in association with a hypercoagulable vasospastic situation leading to microcirculatory occlusion. The ischemic changes begin distally and may advance proximally to involve a whole extremity. The pathogenesis of SPG may involve the Schwartzman reaction, bacterial endotoxin release, and platelet plugging in peripheral arterioles due to vascular collapse and DIC. [10] A more or less prototypical clinical presentation of SPG in spite of a large number of etiological associations is suggestive of DIC as the final common pathway of its pathogenesis. [11] Existing data showed that DIC might be associated with 85 to 100% of cases of SPG. [3],[6],[12] DIC most commonly occurs due to sepsis [2],[3],[6] and pneumococcus is the most common organism responsible. [6] In case of septicemia, activation of neutrophils and release of vasoactive substances may also play a contributing role.
Cold-induced vasospasm may be an additive factor in the causation of gangrene. In a recent prospective study, most of the patients of SPG (10 out of 14 patients) were seen during winter season. [6] The patients of SPG may be either healthy or immunosupressed prior to the onset of this syndrome. SPG is associated with a wide gamut of causative factors [Table - 1].
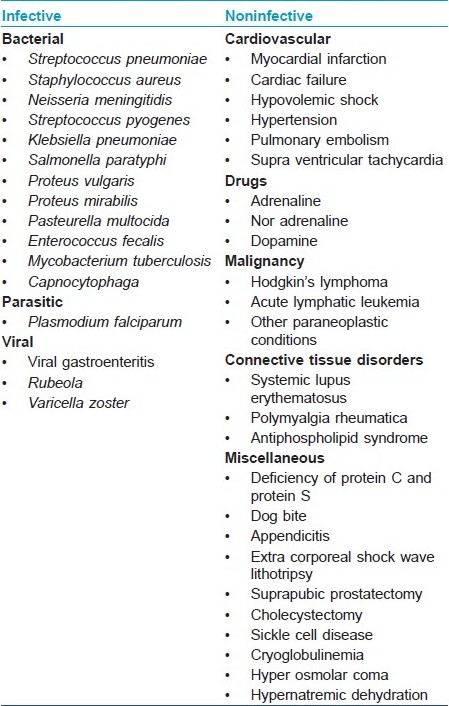
Infective organisms such as Pneumococcus, [6],[13] Staphylococcus aureus,[3],[6] Neisseria meningitides,[3] Streptococcus pyogenes,[6] Klebsiella pneumoniae,[6],[14] E. coli,[6] Salmonella paratyphi, Proteus vulgaris, Proteus mirabilis, Pusturella multocida,[15] Pseudomonas, [3],[6] Enterococcus faecalis,[16] Capnocytophaga,[3] Plasmodium falciparum, [17],[18] Mycobacterium tuberculosis, [19] Rubeola virus, and Varicella zoster virus [1],[15] have been implicated as the causative agents of SPG. Viral gastroenteritis has also been described as a causative factor of SPG. [15]
Noninfective causes of SPG include myocardial infarction, [15] cardiac failure, [1],[15] hypovolemic shock, [1] hypertension, [1] pulmonary embolism, [15] supraventricular tachycardia, Hodgkin′s lymphoma, [12] systemic lupus erythematosus, [20] polymyalgia rheumatica, [12] decreased levels of protein C and protein S, [21] antiphospholipid antibodies, [14] cryoglobulinemia, acute lymphatic leukemia, [16] dog bite, [3] appendicitis, [15] extra corporeal shock wave lithotripsy, [22] suprapubic prostatectomy, [23] cholecystectomy, [6] sickle cell disease, [20] hyper osmolar coma, [15] hypernatremic dehydration, small cell lung cancer. [24] Drugs [15] like adrenaline, noradrenaline, [25] and dopamine [26] may also be important causative factors. Drugs like ergotamine and vasopressin can cause vasospastic Raynaud′s phenomena and are better excluded from the etiology of SPG. [15] Asplenia, immunosupression, diabetes mellitus, and renal failure are amongst the other aggravating factors. [27]
Clinical and Demographic Features
The exact incidence of SPG is not known. Patients of any age group can be affected. Davis et al reported a slight male preponderance for the disease (7 out of the 12 patients). [3] On the other hand, in a series by the present authors, females outnumbered males (9 out of 14). [6] Tiwari et al. also found slight female preponderance in their study. [7] Fever and pain in the extremities may be followed by other constitutional symptoms. SPG should be suspected at the first sign of marked coldness, pallor, and cyanosis of the acral parts of the body, as the condition can progress rapidly to acrocyanosis and, if not reversed, frank gangrene. [8],[20] The gangrene is of dry type, associated with mummification [Figure - 1], and often leads to amputation. The gangrenous affection is frequently symmetric. Infection is usually absent in the lesional skin. SPG is associated with intact distal pulses as large vessels are spared. [20] The upper and lower extremities, tip of the nose, borders of the ears, genitalia, and scalp are the areas of predilection. [1],[8] Purpuric patches leading to skin necrosis (purpura fulminans) are often found on different body parts [Figure - 2]. Features of shock are also present in a good number of cases. [3],[6]
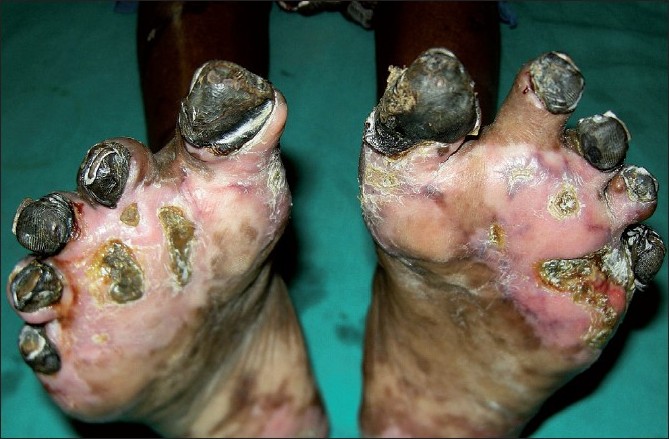 |
| Figure 1: Symmetrical peripheral gangrene of the toes with resorption of the digits |
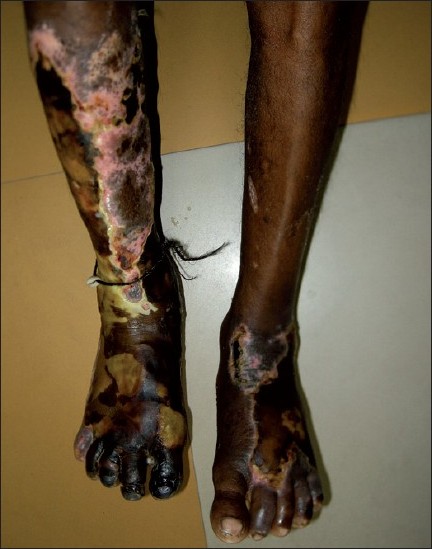 |
| Figure 2: Peripheral gangrene with purpura fulminans acutely developing in patient with septicemia |
History regarding any recent drug intake, surgical procedure, or any systemic illness should be taken and a thorough systemic examination should be performed to find out any infective focus, presence of any internal malignancy, collagen vascular disease, or cardiovascular disease.
The clinical manifestations of DIC may often accompany the gangrene. [16]
Investigations
The goal of laboratory investigation [Table - 2] is to establish the diagnosis of SPG and to find out the underlying predisposing factors. Microangiopathic changes (e.g. presence of schistocytes) on peripheral blood smear should particularly be sought as it may be an indicator of DIC. Peripheral blood smear is also useful for the detection of malarial parasites. Laboratory features of DIC may be absent in the early course of the disease but may evolve later in the course of the disease. [3],[6] Thus, repeated screening for features of DIC should be undertaken. A sudden increase in serum lactate to high levels with bluish discoloration of the acral areas often heralds the onset of SPG. [11]

Peripheral Doppler ultrasound examination shows sparing of the large peripheral arteries. [6] The histopathology of cutaneous biopsy specimens is usually nonspecific and nondiagnostic. [3],[6] Microthrombi in the lumen of the capillaries of the superficial and deep vascular plexus with intraluminal deposition of fibrin and subtle extravasation of red blood cells, and subepidermal cell-poor bulla [3] may be found in the biopsy specimen. Neither vasculitis nor inflammatory infiltrates are observed on the vascular walls. [15]
Differential Diagnosis
Other causes of acral gangrene must be ruled out to establish a diagnosis of SPG.
Thromboangitis obliterans, atherosclerosis, thromboembolic gangrene, secondary Raynaud′s phenomenon, diabetes, neuropathy, chemical or toxic agents, calciphylaxis, and vasculitic gangrene are close mimickers of the condition amongst others. Sparing of the major arteries, suggestive history and natural course, and absence of features of vasculitis in histopathology should differentiate SPG from these conditions.
Prognosis
SPG is associated with a high rate of amputation (auto-amputation or surgical amputation) of the limbs in the survivors. In a prospective case series from eastern India, six patients had amputation out of the nine survivors of SPG. [6] In another retrospective case series from USA, eight patients had amputation out of the nine survivors. [3] The mortality rate of SPG is also very high. About one third of the patients of different case series had died. [3],[6] In another study from India, one patient died out of the ten affected. [7] Most of the deaths associated with SPG occurred within 5 to 21 days of the onset of gangrene. [6] Leucopenia, in particular, is a poor prognostic factor of SPG, reported to be associated with a high mortality rate. [6] Thus, the development of SPG is an ominous prognostic sign, particularly in the background of DIC.
Role of a Dermatologist
Dermatologists may play a vital role in the recognition of the condition as SPG, to distinguish SPG from other forms of gangrene, to identify the associated important skin lesions (e.g. purpura fulminans), and to assist in the management of the condition. [3],[6]
The patient must be referred to an internist or a critical care specialist for the assessment of hemodynamic status, organ dysfunction, and to find out any source of infection or any other precipitating factor. The treatment of DIC and organ dysfunction is primarily the domain of the internists or critical care specialists. In addition, a dermatologist may be associated with the long-term care of the patient regarding the care of the amputation stumps. [3],[6]
Treatment
Therapeutic options [Table - 3] are largely anecdotal and are not based on controlled clinical trials. No specific treatment has been shown to consistently prevent progression or to reverse the gangrene. A multi-disciplinary team involving internist, critical care specialist, surgeon, and dermatologist should undertake the management of SPG. The patient should ideally be treated in an intensive care unit.
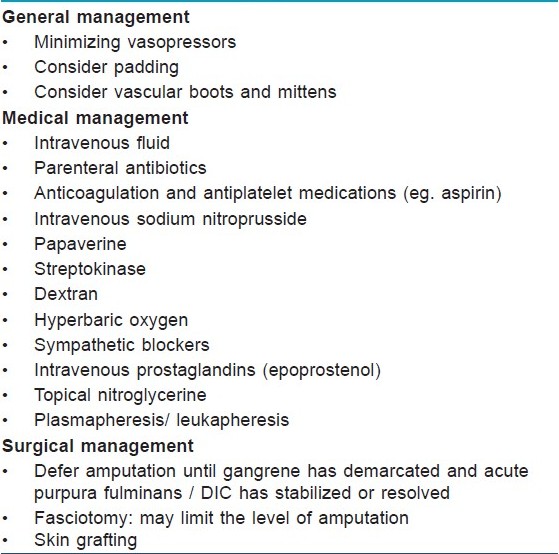
Identification and treatment of the underlying cause is the most important part of the treatment. Vasopressor agents, commonly used in the management of sepsis-induced hypotension, may aggravate this condition. [15]
Disseminated intravascular coagulation should be corrected as appropriate; its cause should be found out and treated aggressively. [8] Intravenous fluid and appropriate parenteral antibiotics should be started early. [8],[18]
Management of DIC should be guided by basic tests of coagulation. [3] If bleeding is the predominant feature, depleted coagulation factors are replaced. On the other hand in cases where thrombosis is predominant, several anticoagulants are tried. Use of heparin has not been proven to improve survival. Randomized trials failed to show any encouraging results regarding use of antithrombin. Recombinant activated protein C, plasmapheresis, intravenous immunoglobulin, continuous plasma ultrafiltration, and continuous veno-veno hemofiltration have also been used with variable success rate. [3]
Other measures that might be helpful are sympathetic blockade in the form of ganglion block or intravenous trimethaphan therapy, [8] intravenous nitroprusside therapy, [28] topical nitroglycerine ointment, [27] local or intravenous infusion of an a-blocker (phentolamine, chlorpromazine), [6] and intravenous infusion of prostaglandin (epoprostenol). [29] Papaverine, reserpine, streptokinase, dextran, and hyperbaric oxygen therapy have not been shown to be beneficial. [8],[27] The addition of oral corticosteroids is also not of help. [30] Rarely, treatment has been shown to prevent progression or to reverse incipient gangrene. [30] Inter-digital padding and protection from trauma may also decrease tissue injury. [15] Amputation of the gangrenous areas may be inevitable, but initially a nonsurgical approach to management is preferred to allow time for the patient′s condition to stabilize and to allow the gangrene to become demarcated. [27],[30]
Later on, skin grafting may be needed. The degree of gangrene may be less extensive than expected initially. Meticulous, supportive management is also of paramount importance. Early physiotherapy may restore joint mobility and range of motion. [15]
Conclusion
SPG is a clinical syndrome of sinister prognostic implication in terms of loss of life or limbs. DIC is an almost universal finding and is probably the final common event in the microvascular insult that gives rise to the prototypical clinical features of this syndrome. The rarity of this condition has precluded controlled clinical trials; identification and treatment of underlying etiological factors and treatment of DIC have remained the mainstays of management. Awareness of the condition and prompt recognition may go a long way in early institution of multidisciplinary care for the patients. Further works, preferably collaborative multicenter studies, are needed to clarify the pathogenesis and formulate rational treatment guidelines.
| 1. |
McGouran RC, Emmerson GA. Symmetrical peripheral gangrene. Br Heart J 1977;39:569-72.
[Google Scholar]
|
| 2. |
Kalajian AH, Turpen KB, Donovan KO, Malone JC, Callen JP. Phenylephrine-induced microvascular occlusion syndrome in a patient with a heterozygous factor V Leiden mutation. Arch Dermatol 2007;143:1314-7.
[Google Scholar]
|
| 3. |
Davis MD, Dy KM, Nelson S. Presentation and outcome of purpura fulminans associated with peripheral gangrene in 12 patients at Mayo Clinic. J Am Acad Dermatol 2007;57:944-56.
[Google Scholar]
|
| 4. |
Karunatilaka DH, De Silva JR, Ranatunga PK, Gunasekara TM, Faizal MA, Malavige GN. Idiopathic purpura fulminans in dengue hemorrhagic fever. Indian J Med Sci 2007;61:471-3.
[Google Scholar]
|
| 5. |
Hutchison J. Severe symmetrical gangrene of the extremities. Br Med J 1891;2:8-9.
[Google Scholar]
|
| 6. |
Ghosh SK, Bandyopadhyay D, Ghosh A. Symmetrical peripheral gangrene: A prospective study of 14 consecutive cases in a tertiary-care hospital in eastern India. J Eur Acad Dermatol Venereol 2010;24:214-8.
[Google Scholar]
|
| 7. |
Tiwary SK, Shankar R, Khanna R, Khanna AK. Symmetrical peripheral gangrene. Int J Surg 2006;7:2.
[Google Scholar]
|
| 8. |
Parmar MS. Symmetrical peripheral gangrene: A rare but dreadful complication of sepsis. CMAJ 2002;167:1037-8.
[Google Scholar]
|
| 9. |
Davis MD. Peripheral symmetrical gangrene. Mayo Clin Proc 2004;79:914.
[Google Scholar]
|
| 10. |
Tripathy S, Rath B. Symmetric peripheral gangrene: Catch it early. J Emerg Trauma Shock 2010;3:189-90.
[Google Scholar]
|
| 11. |
Sharma BD, Kabra SR, Gupta B. Symmetrical peripheral gangrene. Trop Doct 2004;34: 2-4.
[Google Scholar]
|
| 12. |
Molos MA, Hall JC. Symmetrical peripheral gangrene and disseminated intravascular coagulation. Arch Dermatol 1985;121:1057-61.
[Google Scholar]
|
| 13. |
Johansen K, Hansen ST Jr. Symmetrical peripheral gangrene (purpura fulminans) complicating pneumococcal sepsis. Am J Surg1993;165:642-5.
[Google Scholar]
|
| 14. |
Yoo JH, Min JK, Kwon SS, Jeong CH, Shin WS. Symmetrical peripheral gangrene complicating Klebsiella pneumoniae sepsis associated with antiphospholipid antibodies. Ann Rheum Dis 2004;63:459-60.
[Google Scholar]
|
| 15. |
Kashyap R, Behl RK, Mahajan S, Jaret P, Patial RK, Kaushal SS. Symmetrical peripheral gangrene due to viral gastroenteritis. J Assoc Physicians India 2004;52:500-1.
[Google Scholar]
|
| 16. |
Pedraz J, Delgado-Jiménez Y, González-De Arriba A, Ríos-Buceta L, Fernández-Herrera J, García-Diez A. Peripheral symmetrical gangrene. Clin Exp Dermatol 2009;34:552-4.
[Google Scholar]
|
| 17. |
Bhattacharyya PC, Agarwal JP , Sharma M. Symmetric peripheral gangrene of the lower limbs in a case of complicated falciparum malaria. Southeast Asian J Trop Med Public Health 2008;39:589-92.
[Google Scholar]
|
| 18. |
Kakati S, Doley B, Barman B, Devi A. Symmetric peripheral gangrene and falciparum malaria. J Assoc Physicians India 2004;52:498-9.
[Google Scholar]
|
| 19. |
Itin P, Stalder H, Vischer W. Symmetrical peripheral gangrene in disseminated tuberculosis. Dermatologica 1986;173:189-95.
[Google Scholar]
|
| 20. |
Diwan AG, Mittal J, Jadhav A, Pradhan AB. Symmetrical peripheral gangrene with multifactorial origin. J Indian Acad Geriatr 2005;3:103-9.
[Google Scholar]
|
| 21. |
Agrawal A, Rastogi A, Tiwari D. Symmetric peripheral gangrene with mixed malaria. Indian J Pediatr 2007;74:587-8.
[Google Scholar]
|
| 22. |
Mellor SJ, Cheshire NJ. Symmetrical peripheral gangrene after extracorporeal shockwave lithotripsy. BJU Int 1999;84:167-8.
[Google Scholar]
|
| 23. |
Crisci A, Giannarini G, Selli C. Symmetrical peripheral gangrene after suprapubic prostatectomy. Scand J Urol Nephrol 2002;36:478-80.
[Google Scholar]
|
| 24. |
Arrowsmith JE, Woodhead MA, Bevan DH, Nanson EM, Cummin AR. Digital gangrene in small cell lung cancer: Response to aspirin treatment. Thorax 1991;46:63-4
[Google Scholar]
|
| 25. |
Hayes MA, Yau EH, Hinds CJ, Watson JD. Symmetrical peripheral gangrene: Association with noradrenaline administration. Intensive Care Med 1992;18:433-6.
[Google Scholar]
|
| 26. |
Colak T, Erdogan O, Yerebakan O, Arici C, Gurkan A. Symmetrical peripheral gangrene and dopamine. Ulus Travma Derg 2003;9:222-4.
[Google Scholar]
|
| 27. |
Avasthi R, Chaudhary SC, Singh KP, Makker JS. Symmetrical peripheral gangrene. J Assoc Physicians India 2008;56:442.
[Google Scholar]
|
| 28. |
Joynt G, Doedens L, Lipman J, Bothma P. High-dose adrenalin with low systemic vascular resistance and symmetrical peripheral gangrene. S Afr J Surg 1996;34:99-101.
[Google Scholar]
|
| 29. |
Denning DW, Gilliland L, Hewlett A, Hughes LO, Reid CD. Peripheral symmetrical gangrene successfully treated with epoprostenol and tissue plasminogen activator. Lancet 1986;2:1401-2.
[Google Scholar]
|
| 30. |
Davis MP, Byrd J, Lior T, Rooke TW. Symmetrical peripheral gangrene due to disseminated intravascular coagulation. Arch Dermatol 2001;137:139-40.
[Google Scholar]
|
Fulltext Views
9,598
PDF downloads
2,941





