Translate this page into:
Systemic T cell lymphoma presenting as cutis verticis gyrata
Correspondence Address:
Susanne Pulimood
Department of Dermatology, Venereology and Leprosy,Christian Medical College, Vellore - 632 004, Tamil Nadu
India
| How to cite this article: George AA, George L, Mahabal G, Bindra M, Pulimood S. Systemic T cell lymphoma presenting as cutis verticis gyrata. Indian J Dermatol Venereol Leprol 2015;81:631-633 |
Sir,
A 34-year-old man, presented with progressive swelling and serous discharge on his face and scalp for 4 months. General examination revealed pallor and multiple, enlarged, non-tender, discrete, mobile lymph nodes in bilateral cervical, axillary, and inguinal regions, the largest of which was 5 cm × 6 cm in the right axillary region, associated with hepatosplenomegaly. He had a leonine facies. The skin of the scalp was thrown into cerebriform folds with diffuse loss of hair [Figure - 1]a. There was generalized xerosis with a few scattered infiltrated nodules [Figure - 1]b on the trunk and extremities ranging in size from 1-2 cm and diffuse scaling over the palms and soles. The differentials considered were lymphedema secondary to a lymphatic obstruction, leukemoid/lymphomatous infiltration and sarcoidosis. On hematological examination, he was found to have anemia (10.8 g/dl) and leukocytosis (63.4 × 109/L) associated with a serum lactate dehydrogenase of 1110 U/L. Serology for Epstein-Barr virus and human T-lymphotrophic virus were negative. Skin biopsies done from the scalp and a nodule on the back showed mild lamellar hyperkeratosis, focal parakeratosis and irregular acanthosis of the epidermis. There was a pan-dermal, moderate to dense infiltrate of medium sized lymphoid cells with vesicular nuclei, irregular nuclear membranes, small nucleoli and scant cytoplasm. Numerous apoptotic bodies were seen [Figure - 2]a and [Figure - 2]b. On immunohistochemistry, these cells were positive for CD3 [Figure - 3]a, CD4 [Figure - 3]b, focally positive for CD25 [Figure - 3]c and negative for CD5, CD7, CD20, CD34 and terminal deoxynucleotidyl transferase. The MIB-1 proliferation index [Figure - 3]d was 85%. Lymph node biopsy from the right axilla was consistent with high grade T-cell lymphoma on histopathology and immunohistochemistry [Figure - 4]. Bone marrow biopsy showed myeloid hyperplasia with increased eosinophils and its precursors. T cell receptor re-arrangement studies demonstrated clonality for the alpha-beta type. CD 3 and CD 4 positivity indicated a T cell lymphoma. CD 20 negativity ruled out a B cell lymphoma. CD 7, CD 34 and terminal deoxynucleotidyl transferase negativity ruled out a T cell lymphoblastic leukemia/lymphoma. CD 25 positivity is usually seen in adult T cell lymphoma, which was ruled out in view of the human T lymphotropic virus serology being negative. A high MIB-1 proliferation index indicated a high grade lymphoma. On this basis the diagnosis of high grade T cell non-Hodgkin lymphoma was made. He was due to be started on cyclophosphamide, hydroxydaunorubicin, oncovin, prednisolone chemotherapy regimen, but was lost to follow-up as he wished to continue treatment in his home town.
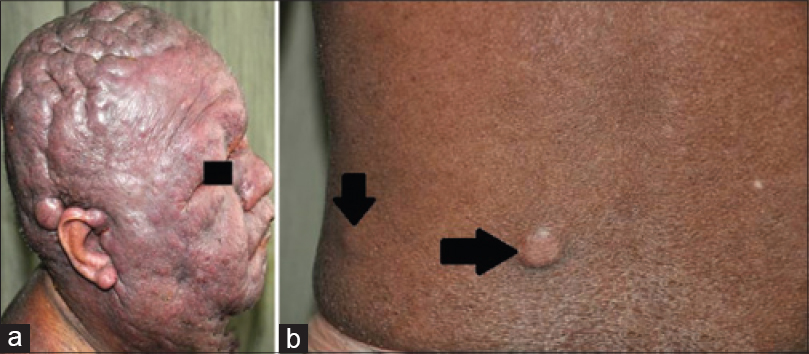 |
| Figure 1: (a) Cutis verticis gyrata with leonine facies, (b) infiltrated nodule on the back |
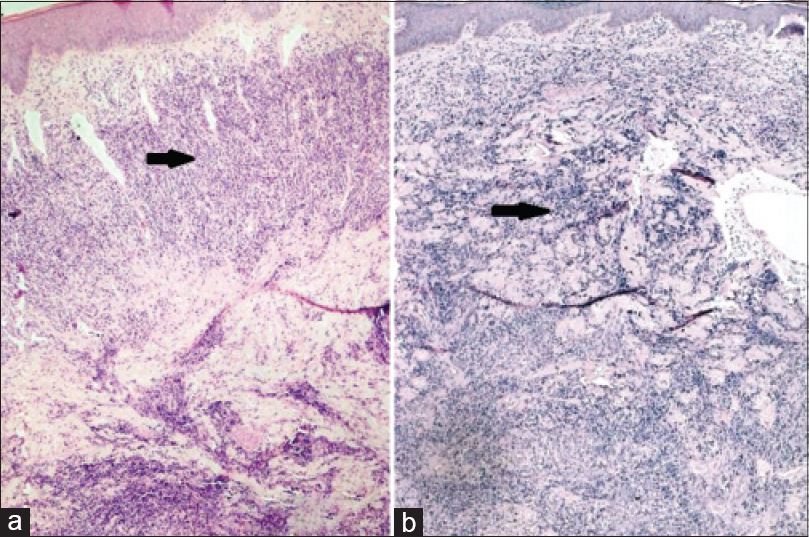 |
| Figure 2: (a) Biopsy from the scalp showing moderate to dense infiltrate of medium sized atypical lymphoid cells in the dermis (H and E stain, ×5), (b) biopsy from the nodule on the back showing pandermal infiltrates of atypical lymphoid cells (H and E stain, ×5) |
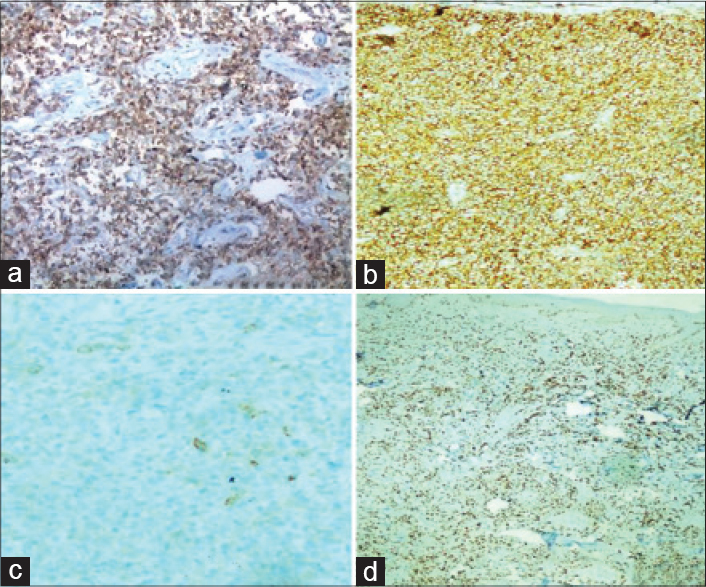 |
| Figure 3: (a) Tumor cells which are CD3 positive on immunohistochemistry (×20), (b) tumor cells which are CD 4 positive on immunohistochemistry (×20), (c) tumor cells which are focally CD 25 positive on immunohistochemistry (×20), (d) tumor cells with a MIB index of 80% (×20) |
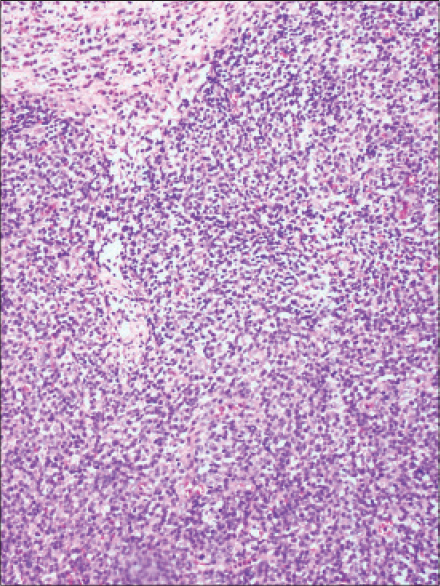 |
| Figure 4: Biopsy from the lymph node showing complete effacement of architecture by sheets of medium sized atypical lymphoid cells (H and E stain, ×10) |
Cutis verticis gyrata is a rare condition wherein the skin on the scalp is redundant, thickened, and thrown into folds forming deep furrows and convoluted ridges, resembling the gyri and sulci of the cerebral cortex. This condition was originally described in 1837 and classified in the mid 1900s into primary and secondary. The primary form which is characterized by an essentially normal histopathology of the skin is further divided based on the absence or presence of underlying neurological and ophthalmological manifestations into essential and non-essential respectively. Secondary cutis verticis gyrata has been reported to occur secondary to endocrine diseases (acromegaly, myxedema, Grave's disease, diabetes mellitus), hereditary disorders (pachydermoperiostosis, Turner syndrome, Noonan syndrome, Ehlers-Danlos syndrome, Beare-Stevenson syndrome, Fragile X syndrome, Klinefelter syndrome, “Michelin tire baby” syndrome, tuberous sclerosis, hyper IgE syndrome), inflammatory cutaneous diseases (eczema, psoriasis, Darier's disease, folliculitis, impetigo, erysipelas, atopic dermatitis, acne conglobata, acanthosis nigricans), infections (syphilis), benign tumors/infiltrates (dermatofibroma, cerebriform intradermal naevus, neurofibroma, neurinoma, collagenoma, focal mucinosis, amyloidosis, intraventricular ependymoma, cutaneous leiomyomatosis, cutaneous neurocristic hamartoma) and malignant tumors (fallopian tube carcinoma, infiltrating ductal carcinoma, angiosarcoma, malignant melanoma, acute myelogenous leukemia, acute monoblastic leukemia).[1],[2] We found two previous reports of cutis verticis gyrata secondary to haematological malignancies. The first was a 28-year-old man with acute myelogenous leukemia who developed cutis verticis gyrata as a result of chloromatous involvement of the scalp.[3] The other was a 64-year-old man with acute monoblastic leukemia who developed cutis verticis gyrata in the terminal phase of his disease.[4] Both patients succumbed to their disease. Our patient with high grade T cell lymphoma was also found to have cutis verticis gyrata in the advanced stage of his disease, but interestingly this was the cutaneous manifestation atfirst presentation to the clinic.
Cutaneous lesions have been reported in 50% of patients with non-Hodgkin lymphoma.[5] The cutaneous lesions in systemic non-Hodgkin lymphoma have been classified by Epstein and MacEachern into specific and non-specific. Specific lesions are those which on histopathology yield characteristic malignant cells which aid in the diagnosis of the lymphoma. Non-specific lesions are those that do not yield a characteristic histopathology. Specific lesions have been reported in 15–20% of patients with systemic non-Hodgkin lymphoma.[5] These lesions include macules, infiltrated plaques, papulo-nodules, ulcerative masses and erythroderma. Specific lesions largely occur in late stage disease due to the presence of neoplastic cells in the peripheral circulation and are thus associated with a poorer prognosis.[5] We were unable to find any previous reports of cutis verticis gyrata presenting as a specific lesion in non-Hodgkin lymphoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Yang JJ, Sano DT, Martins SR, Tebcherani AJ, Sanchez AP. Primary essential cutis verticis gyrata – Case report. An Bras Dermatol 2014;89:326-8.
[Google Scholar]
|
| 2. |
Marque M, Gardie B, Bressac de Paillerets B, Rustin P, Guillot B, Richard S, et al. Novel FH mutation in a patient with cutaneous leiomyomatosis associated with cutis verticis gyrata, eruptive collagenoma and Charcot-Marie-Tooth disease. Br J Dermatol 2010;163:1337-9.
[Google Scholar]
|
| 3. |
Cheson BD, Christiansen RM. Cutis verticis gyrata: unusual chloromatous disease in acute myelogenous leukemia. Am J Hematol 1980;8:415-8.
[Google Scholar]
|
| 4. |
Passarini B, Neri I, Patrizi A, Masina M. Cutis verticis gyrata secondary to acute monoblastic leukemia. Acta Derm Venereol 1993;73:148-9.
[Google Scholar]
|
| 5. |
Carlesimo M, Narcisi A, Rossi A, Saredi I, Orsini D, Pelliccia S, et al. Cutaneous manifestations of systemic non-Hodgkin lymphomas (NHL): study and review of literature. J Eur Acad Dermatol Venereol 2014;28:133-41.
[Google Scholar]
|
Fulltext Views
2,772
PDF downloads
955





