Translate this page into:
The additional effect of 5% atorvastatin shampoo in the treatment of adult patients with mild to moderate seborrheic dermatitis of the scalp: A prospective, randomised, double-blind trial
Corresponding authors: Dr. Sara Samadi, Department of Internal Medicine, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. samadi.srh@gmail.com and Dr. Mohammad Javad Yazdanpanah, Cutaneous Leishmaniasis Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. yazdanpanahmj@mums.ac.ir
-
Received: ,
Accepted: ,
How to cite this article: Miri F, Sadeghi M, Abbaspour M, Samadi S, Yazdanpanah MJ. The additional effect of 5% atorvastatin shampoo in the treatment of adult patients with mild to moderate seborrheic dermatitis of the scalp: A prospective, randomised, double-blind trial. Indian J Dermatol Venereol Leprol. 2025;91:222-5. doi: 10.25259/IJDVL_245_2024
Abstract
Background
Seborrheic dermatitis (SD) is a long-lasting inflammatory skin condition that predominantly impacts regions abundant in sebaceous glands, including the scalp.
Objectives
To assess the efficacy and anti-inflammatory effect of atorvastatin as an additive treatment among SD patients.
Methods
In a prospective, randomised, double-blind trial, 46 patients over 18 years old with mild to moderate scalp SD were randomly assigned to receive either 2% ketoconazole shampoo or 2% ketoconazole shampoo plus 5% atorvastatin. The severity of dermatitis was assessed based on the symptom scale of seborrheic dermatitis (SSSD), and the variables of erythema, scaling, and itching, at baseline and 4 weeks after the intervention.
Results
Based on our analyses, both treatment methods significantly reduced the SSSD scores. However, the average SSSD score in patients using ketoconazole shampoo plus atorvastatin decreased by an average of five points after 1 month. This reduction was comparable to the average decline of 3.5 points observed in the group using ketoconazole shampoo alone. Specifically, the severity of dermatitis, as assessed by the SSSD score, significantly decreased by 1.92 points more, in individuals using the atorvastatin-containing shampoo compared to the comparison group (P = 0.02).
Limitation
This research was conducted at a single centre which limits the validity of the findings.
Conclusion
The results of this study suggest that shampoo containing atorvastatin provides a statistically significant effect compared to ketoconazole shampoo alone, indicating its potential as an alternative treatment for SD. The treatment notably alleviates symptoms associated with scaling and itching which are the common manifestations of the condition.
Keywords
Anti-dandruff shampoos
antifungal
anti-inflammatory
atorvastatin
seborrheic dermatitis
Introduction
Seborrheic dermatitis (SD) is a chronic inflammatory skin condition that affects areas rich in sebaceous glands, such as the scalp.1 It is found in approximately 3% of the population with a higher occurrence among young men than women.2 Although the exact cause of SD is not clear, the colonisation of the fungus Malassezia and the inflammatory immune system response to this fungus play a significant role in the pathology of this condition.3
Recently, evidence has emerged regarding the anti-inflammatory and antifungal properties of statins.4 Statins could potentially be beneficial in addressing inflammatory skin conditions, including acne, vitiligo and dermatitis.5
The current double-blind, randomised trial focuses on investigating the efficacy of atorvastatin and assessing the alterations in the severity of SD patients.
Methods
Study design
This study was conducted as a prospective, randomised, double-blind clinical trial in which both patients and trial investigators were blinded. This clinical trial was conducted during the period from 2021 to 2022 on patients with mild to moderate SD of the scalp. Overall, 46 participants were divided into two groups using block randomisation; one group received ketoconazole shampoo alone (23 individuals), and the other group received ketoconazole shampoo plus 5% atorvastatin (23 individuals) [Figure 1]. Furthermore, a research assistant distributed the shampoos to both groups and did not intervene in any other trial phases. The control group received 2% ketoconazole shampoo twice a week for 4 weeks, with each application leaving the shampoo on the scalp for 5 minutes. The intervention group used 2% ketoconazole shampoo plus 5% atorvastatin shampoo twice a week for 4 weeks, with each application leaving the shampoo on the scalp for 5 minutes. Both ketoconazole and atorvastatin shampoos were provided by the pharmaceutical company in standardised bottles with coded solutions added to baby shampoo.
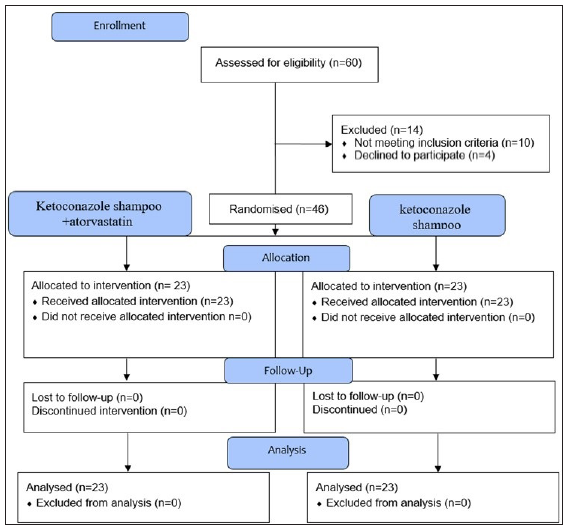
- Flow diagram of the trial.
Sampling
The inclusion criteria for participants in the study were: (1) age between 18 and 65; (2) clinical diagnosis of mild to moderate SD, based on the Symptom Scale of Seborrheic Dermatitis (SSSD) assessed by the same clinician; (3) no topical or systemic treatment for the condition in the past month; and (4) no exacerbating disorders such as HIV or Parkinson’s disease. The exclusion criteria from the study were as follows: (1) patients with severe SD, with a severity index (SI) score higher than 10; (2) pregnancy or breastfeeding or becoming pregnant during the treatment; (3) lack of willingness for treatment; (4) Inability to continue the treatment; and (5) use of anti-dandruff shampoos during the treatment.
The primary measure of interest was the modification in the severity of SD, evaluated by the SSSD criteria. The severity of the condition in each participant was assessed using the SSSD criteria at two time points: at baseline and 4 weeks after treatment. The scores were examined based on the severity of three main symptoms of SD: erythema, scaling, and itching. Erythema and scaling were scored on a scale ranging from 0 to 5, where 0 indicated no signs of scaling or erythema, 1 represented minimal or initial signs, 2 indicated a mild scaling or erythema, 3 assigned moderate scaling or erythema, 4 represented severe scaling or erythema, and 5 indicated very severe symptoms.6 Itching severity was assessed using a visual analogue scale (VAS) ranging from 0 (no itch) to 100 (worst possible imaginable itch). The VAS scores were divided into six categories using the following criteria: scores of ≤10 mm were assigned a value of 0, 11–20 mm were assigned a value of 1, 21–40 mm were assigned a value of 2, 41–60 mm were assigned a value of 3, 61–80 mm were assigned a value of 4 and scores ranging from 81 to 100 mm were assigned a value of 5. The clinical severity of SD was assessed by calculating the total scores, which were then categorised into severity indices as follows: mild (0–6), moderate (7–9), and severe (10–15).7,8
Assessment of adverse effects
To assess the potential adverse effects of the medications, patients were questioned at each visit regarding the occurrence of itching, burning, erythema, or exacerbation of pre-existing symptoms.
Statistical analysis
The data was analysed using SPSS software (version 16.0, SPSS Inc., Chicago, IL, USA). The chi-square test and Fisher’s exact test were utilised to analyse the data. The t-test for mean differences and one-way analysis of covariance (ANCOVA) were used for comparing means and determining the intervention effect. P < 0.05 was considered statistically significant.
Results
In the intervention group, there were 23 participants with an average age of 31.3 years. Out of these, 11 participants (47.8%) were males and 12 (52.2%) were females [Table 1]. In the control group, there were 23 participants with an average age of 29 years. Among them, 10 participants (43.5%) were males and 13 (56.6%) were females. The mean SSSD score in the intervention group was 7.78, while it was 6.61 in the control group. In the intervention group, 17.4 (4 participants) and 82.6% (19 participants) had mild and moderate SD, respectively, while in the control group, 47.8 (11 participants) and 52.2% (12 participants) had mild and moderate dermatitis, respectively.
| Characteristics | Atorvastatin + Ketoconazole (n = 23) | Ketoconazole (n = 23) |
|---|---|---|
| Age, year (SD*) | 31.3 (8.3) | 29.0 (9.0) |
| Sex | ||
| Male, n (%) | 11 (47.8) | 10 (43.5) |
| Female n (%), | 12 (52.2) | 13 (56.6) |
| SSSD$, mean (SD) | 7.78 (1.31) | 6.61 (1.67) |
| Disease severity | ||
| Mild, n (%) | 4 (17.4) | 11 (47.8) |
| Moderate, n (%) | 19 (82.6) | 12 (52.2) |
Both treatment approaches have demonstrated a notable effect in reducing inflammation, itching, and scaling. Following the initiation of treatment, the average scores for clinical outcomes, including erythema, itching, and scaling have decreased [Table 2]. A significant reduction in itching and scaling among patients treated with ketoconazole plus atorvastatin shampoo was evident compared to those treated with ketoconazole shampoo alone [Table 3]. The results suggest that the reduction in erythema has been comparable among all patients, and the difference in the mean score between the two groups was statistically insignificant (P = 0.48) [Table 3].
| Clinical outcomes | Atorvastatin | Ketoconazole | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Erythema | ||||
| No signs | 4 (17.4) | 5 (21.7) | 5 (21.7) | 6 (26.11) |
| First signs | 9 (39.4) | 14 (60.9) | 10 (43.5) | 14 (60.9) |
| Mild | 6 (26.1) | 3 (13.0) | 7 (30.4) | 3 (13.0) |
| Moderate | 4 (17.4) | 1 (4.3) | 1 (4.3) | 0 |
| Scaling | ||||
| No signs | 9 (39.1) | 1 (4.3) | 4 (17.4) | 1 (4.3) |
| First signs | 11(47.8) | 14 (60.9) | 11 (47.8) | 9 (39.1) |
| Mild | 3 (13.0) | 6 (26.1) | 7 (30.4) | 11 (47.8) |
| Moderate | 0 | 2 (8.7) | 1 (4.3) | 2 (8.7) |
| Itching (mm) | ||||
| <10 | 5 (21.7) | 15 (65.2) | 2 (8.7) | 9 (39.1) |
| 11–20 | 5 (21.7) | 8 (34.8) | 5 (21.7) | 11 (47.8) |
| 21–40 | 7 (30.4) | 0 | 5 (21.7) | 3 (13.0) |
| 41–60 | 6 (26.1) | 0 | 5 (34.8) | 0 |
| 61–80 | 0 | 0 | 3 (13.0) | 0 |
| Disease severity based on the SSSD* score | ||||
| Mild | 4 (17.4) | 22 (95.7) | 11 (47.8) | 23 (100.0) |
| Moderate | 19 (82.6) | 1 (4.3) | 12 (52.2) | 0 |
SSSD*: Symptom scale of seborrheic dermatitis
| Treatment | Erythema, mean score ± SE* | Scaling, mean score ± SE* | Itching, mean score ± SE* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After | Mean differences | P | Baseline | After | Mean differences | P | Baseline | After | Mean differences | P | |
| Atorvastatin + ketoconazole | 1.4 ± 0.21 | 1.00 ± 0.15 | −0.43 | 0.005 | 3.74 ± 0.14 | 1.39 ± 0.15 | −2.35 | <0.001 | 2.61 ± 0.23 | 0.35 ± 0.10 | −2.26 | <0.001 |
| Ketoconazole | 1.17 ± 0.17 | 0.87 ± 0.13 | −0.30 | 0.02 | 3.22 ± 0.17 | 1.61 ± 0.15 | −1.61 | <0.001 | 2.22 ± 0.25 | 0.74 ± 0.14 | −1.48 | <0.001 |
| Atorvastatin vs. ketoconazole | −0.13 | 0.48 | −0.74 | <0.001 | −0.78 | 0.01 | ||||||
SE*: Standard error
Furthermore, both treatment methods have significantly reduced the SSSD scores [Table 4]. In the group that used shampoo containing atorvastatin and ketoconazole, the average score decreased by 5 points after 1 month. Similarly, in individuals who used shampoo containing ketoconazole alone, the average score decreased by 3.5 points after 1 month.
| Treatment | Baseline | After | Difference in SSSD* score | P |
|---|---|---|---|---|
| Atorvastatin + ketoconazole | 7.78 ± 1.31 | 2.73 ± 1.4 | −5.04 | <0.001 |
| Ketoconazole | 6.61 ± 1.67 | 3.13 ± 1.3 | −3.48 | <0.001 |
| Atorvastatin vs. ketoconazole | −1.92 | 0.02 | ||
SSSD*: Symptom scale of seborrheic dermatitis
In addition, individuals who used shampoo containing atorvastatin and ketoconazole experienced more substantial changes in the SSSD scores compared with those who used ketoconazole alone. Specifically, the severity of dermatitis, as measured by the above scores, exhibited a statistically significant reduction of 1.92 points greater in individuals using atorvastatin-containing shampoo compared to the comparison group (P = 0.02). In addition, no significant adverse effects were reported in this study.
Discussion
Our research demonstrates that the topical application of atorvastatin, in the form of a shampoo, has a significant impact in the treatment of mild to moderate SD and scalp scaling in adults. In line with these findings, the results of a double-blind clinical trial involving 86 patients with mild to moderate scalp SD who were randomly assigned to receive either 5% atorvastatin lotion or 0.1% betamethasone lotion for 4 weeks indicate that topical application of atorvastatin yields results comparable to betamethasone.8 In addition, in a randomised, double-blind, placebo-controlled study with 130 individuals undergoing treatment for hand eczema, participants were randomly assigned to 1% betamethasone ointment with 5% atorvastatin cream or 1% betamethasone ointment with a placebo cream twice daily for 10 days. Individuals in both groups experienced a decrease in mean hand eczema severity index scores as well as average visual analogue scores following the intervention, although the reduction in the hand eczema severity index and visual analogue scores was more pronounced in the atorvastatin group.9
Several investigations have been carried out to explore the anti-inflammatory properties of topical atorvastatin in specific skin conditions.10–13
The clinical pilot study aimed to assess the impact of ointments containing 1% simvastatin-acid sodium salt or 1% atorvastatin calcium salt on vitiligous lesions in patients with non-segmental vitiligo revealed that the use of statins was linked to an elevation in the secretion of anti-inflammatory cytokines and a suppression of pro-inflammatory cytokine production.11 A 2019 study conducted in Saudi Arabia evaluated the effectiveness of a nanoemulgel formulation containing atorvastatin (2.5%) for topical application in wound healing. Histopathological analysis revealed notable enhancement in the histological structure of the skin following 21 days of treatment with atorvastatin nanoemulgel.14
Limitations
This research was conducted at a single center, which hinders the generalizability of the findings. In our study, we employed atorvastatin as an adjunct treatment for healing scalp SD. Therefore, it is essential for prospective clinical studies to examine the effects of other topical statins and their potential therapeutic benefits as standalone treatments on clinical outcomes. Additionally, the formulation of topical atorvastatin utilized in our study was elementary, indicating the need for further verifications in future studies.
Our study employed atorvastatin as an adjunct treatment for scalp SD. Therefore, prospective clinical studies are needed to examine the effects of other topical statins and their potential therapeutic benefits as standalone treatments on clinical outcomes. Additionally, the formulation of topical atorvastatin utilised in our study was elementary, indicating the need for further verifications in future studies.
Conclusion
Topical atorvastatin in shampoo form provides a statistically significant effect along with ketoconazole shampoo, suggesting its potential as an alternative treatment for scalp SD. In other words, although the observed clinical difference between study groups is small, this cannot rule out the clinical effect of ketoconazole. Atorvastatin is shown to have a significant impact on symptoms such as scaling and itchiness that are commonly associated with the disease.
Ethical approval
This study was approved by the Institutional Review Board at Mashhad University of Medical Sciences, number IR.MUMS.MEDICAL.REC.1401.185, dated 13.4.2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This work was supported by the Mashhad University of Medical Sciences (Grant number: 4000331).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)–assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)–assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
References
- Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18:13-26. quiz 19–20
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and associated diseases of seborrheic skin in adults. Clin Epidemiol. 2021;13:845-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Malassezia spp carriage in patients with seborrheic dermatitis. J Dermatol. 1999;26:558-61.
- [CrossRef] [PubMed] [Google Scholar]
- Antifungal effects of statins. Pharmacol Ther. 2020;208:107483.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of seborrheic dermatitis: Comparison of sertaconazole 2% cream versus pimecrolimus 1% cream. Ir J Med Sci. 2013;182:703-6.
- [CrossRef] [PubMed] [Google Scholar]
- Establishment of clinical evaluation criteria for scalp seborrheic dermatitis. J Cosmet Dermatol. 2023;22:3042-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of efficacy and safety of atorvastatin 5% lotion and betamethasone 0.1% lotion in the treatment of scalp seborrheic dermatitis. Clin Cosmet Investig Dermatol. 2019;12:267-75.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Beneficial effects of adding topical atorvastatin 5% cream to topical betamethasone 1% ointment on chronic hand eczema. Arch Iran Med. 2020;23:605-13.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of topical atorvastatin for the treatment of pressure ulcers: A randomized clinical trial. Pharmacotherapy. 2014;34:19-27.
- [CrossRef] [PubMed] [Google Scholar]
- The evaluation of vitiligous lesions repigmentation after the administration of atorvastatin calcium salt and simvastatin-acid sodium salt in patients with active vitiligo (EVRAAS), a pilot study: Study protocol for a randomized controlled trial. Trials. 2019;20:78.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Atorvastatin mucoadhesive tablets in the management of recurrent aphthous stomatitis: A randomized clinical study. BMC Oral Health. 2023;23:285.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of the effect of topical atorvastatin solution for the treatment of papulopustular acne. Int J Curr Pharm Res. 2013;5:59-60.
- [Google Scholar]
- Preparation and evaluation of atorvastatin-loaded nanoemulgel on wound-healing efficacy. Pharmaceutics. 2019;11:609.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







