Translate this page into:
The efficacy of azithromycin in pityriasis rosea: A randomized, double-blind, placebo-controlled trial
Correspondence Address:
Deepika Pandhi
B-1/1101, Vasant Kunj, New Delhi - 110 070
India
| How to cite this article: Pandhi D, Singal A, Verma P, Sharma R. The efficacy of azithromycin in pityriasis rosea: A randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol 2014;80:36-40 |
Abstract
Background: Macrolides are prescribed in the treatment of pityriasis rosea despite conflicting results of the limited number of studies evaluating their role in its treatment. Aim: A randomized double-blind placebo-controlled trial was conducted to evaluate the effect of azithromycin on the clinical course of pityriasis rosea. Methods: Seventy patients of pityriasis rosea were given either azithromycin (n = 35) or placebo (n = 35) and were followed-up at 2, 4 and 6 weeks. Pruritus was assessed in both groups using the visual analogue scale (VAS) . Change in the pityriasis rosea severity score (PRSS) and in the VAS were recorded as outcome measures and were compared statistically. Results: The decrease in PRSS from baseline through 2, 4 and 6 weeks within both treatment (P < 0.001) and placebo (P < 0.001) arms was found to be statistically significant; however, this change was not significantly different in the two groups (P = 0.179). Similarly, the decrease in VAS was found to be statistically significant within both groups (P < 0.001); however, the change was comparable between the two groups (P < 0.937). Analysis by Fisher's exact test did not find a significant difference between the two groups for PRSS and VAS. Conclusion: Azithromycin is not effective in pityriasis rosea and the use of macrolides for this disease should not be encouraged in clinical practice.Introduction
Pityriasis rosea is an acute, self-limiting papulo-squamous skin disorder first described in 1798. [1] The skin rash follows a very distinctive pattern. In three-fourths of the cases, a single, isolated oval scaly pink maculae or patch (the "herald" or "mother patch") appears on the body, particularly on the trunk, upper arms, neck or thighs. The rash of pityriasis rosea typically lasts about 5 weeks and resolves by 8 weeks in more than 80% of patients. [2] Some epidemiologic features (seasonal variation and clustering in communities) suggest that pityriasis rosea may be an infectious disease. Reactivation of latent human herpesvirus-6 and human herpesvirus-7 infection have been suggested as the possible etiologic agents. [3]
Azithromycin and erythromycin, share the same mechanism of action, show a near-identical antimicrobial spectrum of action and share many immuno-modulatory and anti-inflammatory effects. [4],[5] However, the pharmacokinetic properties of azithromycin are more favorable when compared with erythromycin. Only a few studies have attempted to evaluate the efficacy of macrolides (erythromycin or azithromycin) in pityriasis rosea. In 2000, Sharma et al. [6] published a study that showed great success with oral erythromycin in inducing resolution of pityriasis rosea in a group of 45 patients. Similarly, Villarama et al. also found erythromycin to be effective in a randomized double-blind control trial (unpublished). [7] In contrast to the above studies, Amer and Fischer, [8] in the year 2006, concluded that azithromycin does not alter the course of pityriasis rosea and subsequently, Rasi et al. [9] also reported failure of erythromycin in clearing pityriasis rosea lesions. Chuh et al. [7] in their comprehensive systematic review of interventions in pityriasis rosea have recommended more randomized controlled trials in particular to investigate the efficacy of oral erythromycin or other macrolide antibiotics. In view of the conflicting results and to further assess the efficacy of the macrolide, azithromycin in pityriasis rosea, we decided to undertake a randomized double blind study.
Methods
A prospective, randomized, double-blinded, placebo-controlled study was conducted from February 2010 to March 2011 at the dermatology outpatient department of an urban hospital in north India. The sample size was determined using two sided Z test. A sample size of 35 per arm including 10% lost to follow-up was calculated to be required to demonstrate a cure rate of 70% with active treatment and 35% cure in the placebo group with 80% power and 5% level of significance. [6],[8]
Study population
Inclusion criteria
After informed consent, 70 consecutive patients with a diagnosis of pityriasis rosea were recruited. Diagnosis of pityriasis rosea was made by two dermatologists in all patients, based on characteristic clinical features. [10]
Exclusion criteria
If either of the dermatologists did not agree with the diagnosis of pityriasis rosea, the patient was not eligible for enrolement. Those with intake of an antibiotic within 2 weeks prior to the diagnosis of pityriasis rosea, a history of intolerance to azithromycin or erythromycin, the presence of lesions for more than 2 weeks and those with the absence of pruritus at the time of diagnosis were also excluded.
Randomized table provided by a statistician for the generation of the randomization sequence was used for group allocation. Two dermatologists assessed the participants for inclusion or exclusion in the study. A third author randomized the participants into Groups A or B (n = 35 in each group). A pharmacy controlled concealment of randomization was carried out. A clinical nurse assigned oral azithromycin and placebo to either Group A or Group B and dispensed test medications to participants. The coded containers and the key for group allocation and computer generated random numbers list were kept in an opaque and sealed envelope in a locked cupboard, to which access was available only to the nurse. Hence, treatment assignment could not be known to the investigators carrying out evaluation or to patients at any time during the trial. Treatment patients received azithromycin, 12 mg/kg/day for 5 days. The maximum daily dose was 500 mg/day of azithromycin; this was given to all of patients weighing ≥40 kg. Placebo patients were administered multivitamin placebo tablets, similar to azithromycin tablets in color, size, shape and taste. The dermatologists not involved in randomization conducted the subsequent clinical assessment.
Clinical profile
A detailed clinical history and complete physical examination were undertaken; standardized case record forms were used for the purpose, which included age, sex, duration of rash, the season during presentation, history of preceding upper respiratory infection, exposure to a patient of pityriasis rosea, pruritus, and herald patch. Complete blood counts and antistreptolysin-O titers were carried out in all patients and venereal disease research laboratory (VDRL)test was performed to exclude secondary syphilis. Digital photographs were taken at presentation and subsequent follow-up visits.
Outcome measures
The major outcome measures were the mean decrease in itch as assessed by the participants using visual analogue scale (VAS) of 1-10, [11],[12] (a primary outcome measure) and reduction in pityriasis rosea severity score (PRSS) as assessed by the medical practitioner (a secondary outcome measure).
To calculate PRSS, two areas were assessed: (1) the head and trunk (T) and (2) the upper and lower extremities (e). The extent of the disease was first assessed with a 0-3 scale (0 = absence of lesions, 1 = 1-9 lesions, 2 = 10-19 lesions, 3 ≥ 20 lesions). To evaluate the severity of the lesions, three target symptoms termed erythema (E), infiltration (I) and scale (S) were assessed according to a scale of 0-3, in which 0 means a complete lack of cutaneous involvement and 3 represents the most severe possible involvement. To calculate the PRSS, the sum of the severity rating for these three main changes was multiplied with the numeric value (N) of the extent of the disease. The formula is: PRSS = Nt (Et + It + St) +Ne (Ee + Ie + Se). The subscript "t" indicates one side of the trunk and the head and the subscript "e" indicates one side of the extremities. [13] Improvement in PRSS was graded as the percentage reduction as follows: minimal, ≤25%; good, 26-50%; very good, 51-75%; >75%, excellent. Other outcome measure was adverse effects of the treatment.
Patients were seen for follow-up at 2, 4 and 6 weeks after enrolling in the study. Standardized data collection at follow-up visits included: change in the PRSS, [14] change in VAS, medication adverse effects and use of other treatments.
Statistical analysis
Data was analyzed using SPSS version 17.0. The statistician who performed data analyses was blinded regarding the identities of treatment groups. Intention to treat analysis was applied in view of three dropouts. Independent sample′s t-test and Chi-square test was used to compare age, duration of disease and gender. Multiple repeated measures ANOVA test, Tukey′s test and Fisher′s exact test were used to compare the efficacy in the two groups.
Results
Clinical profile
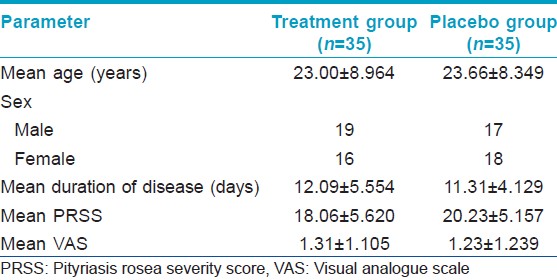
A total of 70 patients were recorded and randomized into treatment (n = 35) and placebo (n = 35) groups. There were three drop outs; two in the treatment and one in the placebo group. Out of 70 patients, 49 had the "classic" papulosquamous lesions, 14 papular lesions and seven papulovesicular lesions. Herald patch was recorded in 14 patients and 10% of patients had lesions over the face. The characteristics of patients in the two groups are depicted in [Table - 1]. The two groups were comparable with respect to age, sex, duration of lesions, PRSS and VAS at baseline.
Outcome measures
PRSS
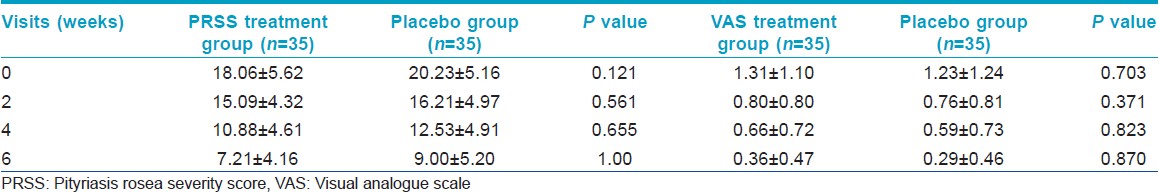
0The mean PRSS in the treatment group at 0, 2, 4 and 6 weeks was 18.06 ± 5.620, 15.09 ± 4.321, 10.88 ± 4.608 and 7.21 ± 4.157 respectively. The mean PRSS in the placebo group at 0, 2, 4 and 6 weeks was 20.23 ± 5.157, 16.21 ± 4.971, 12.53 ± 4.906 and 9.00 ± 5.202 respectively. The decrease in PRSS within both treatment (P < 0.001) and placebo (P < 0.001) arms was found to be significant. In both groups, the fall in PRSS was significant (P < 0.05) at 2 weeks, 4 weeks and 6 weeks. However, the change in PRSS was comparable between the two groups (P = 0.121). A total of 26 patients showed minimal improvement in the PRSS in both groups; while six and nine patients had good improvement in the treatment and placebo group respectively. However, analysis by Fisher′s exact test did not find a significant difference between the two groups for PRSS [Table - 2].
VAS
The mean VAS in the treatment group at 0, 2, 4 and 6 weeks was 1.31 ± 1.105, 0.80 ± 0.797, 0.66 ± 0.725 and 0.36 ± 0.474 respectively. The mean VAS in the placebo group at 0, 2, 4 and 6 weeks was 1.23 ± 1.239, 0.76 ± 0.807, 0.59 ± 0.732 and 0.29 ± 0.456. This decrease in VAS was found to be statistically significant within both groups (P < 0.001). The decrease in VAS was found to be significant (P < 0.05) at 2 weeks. Subsequent fall in VAS at each visit as compared with the previous visit was not significant. Further, change in VAS was comparable between the two groups (P = 0.703). With respect to VAS, the treatment group reported minimal response in eight patients, good in 11 and excellent in five. In the placebo group; five showed a minimal response, good in 11 and very good and excellent in two each. Fisher′s exact test did not find a significant difference between the two groups for VAS [Table - 2].
Complete blood counts were within normal range in all. Antistreptolysin-O was positive in 12 patients of which nine were in treatment and three in the placebo group. There was no significant difference in PRSS values at 0 (P = 0.395), 2 weeks (0.905), 4 weeks (0.645) and 6 weeks (0.236) between the antistreptolysin-O positive patients in the two groups. Similarly, the VAS values at 0 (P = 0.423), 2 weeks (0.655), 4 weeks (0.773) and 6 weeks (1.000) between these patients in the two groups were comparable. There was also no significant difference in the percentage improvement of PRSS and VAS between antistreptolysin-O positive and negative patients (P = 0.220) in the treatment group.
Three patients in the treatment group experienced mild abdominal pain, which did not necessitate drug withdrawal. None in the placebo group experienced any side-effect.
Dicussion
In the 70 patients with pityriasis rosea, a herald patch was seen in 20%, which is consistent with the 12-94% range documented in the literature. [14],[15] Facial lesions were seen in 10%, which has been reported to vary from 15% to 47% of the patients. [15],[16]
The mechanism of action of oral macrolides in pityriasis rosea is unknown. Macrolides are known to have anti-inflammatory and immunomodulatory actions. Their efficacy therefore does not necessarily support bacterial infection(s) or exclude viral infection(s) from being the cause of pityriasis rosea. [7] Sharma et al., in their non-randomized controlled study comprising 45 patients each in the erythromycin and placebo groups claimed a 73.3% cure rate in 2 weeks in the treatment group as compared with none in the placebo group. Pruritus was not considered as a measure of treatment response in their study; indeed, pruritus was not used as an outcome measure in any of the previously published studies evaluating macrolides in pityriasis rosea. [6],[7],[8],[9] Subsequently, in a double blind randomized controlled, unpublished trial, erythromycin was found to be effective as compared to placebo; however, it included only 40 study subjects. [7] In contrast, the favorable results obtained by the above studies could not be confirmed in the present trial with a complete clearance at 6 weeks evident in only one patient each in both groups. The present study measured pruritus on VAS scale and its response to treatment was shown to be similar among the treatment and placebo groups. This is also the first study to use the comprehensive PRSS as compared to the number of lesions as an outcome measure. PRSS is a more precise tool as it takes account of lesional erythema and scaling besides the number of lesions and has been previously used in trials of phototherapy in pityriasis rosea. [13]
There is a dearth of randomized double-blind control trials of macrolides in pityriasis rosea. Amer and Fischer [8] reported the only randomized double-blind placebo controlled published trial, in which azithromycin was not found to be effective in pityriasis rosea. Their study comprised 49 patients and was focused on pediatric age group (mean age = 8 years) while the present study comprised a larger sample size of 70 and included patients with a mean age of 23.3 (range: 2-44 years). The physicians who diagnosed pityriasis rosea in the study by Amer and Fischer [8] were not dermatologists while in the present study the diagnosis was confirmed by a minimum of two dermatologists. Recently, Rasi et al. [9] in a non-randomized, double-blind placebo-controlled study on 184 patients also did not find erythromycin to be effective in pityriasis rosea.
There have been several recent published case reports reporting successful treatment of pityriasis rosea with various macrolides including clarithromycin and roxithromycin. [17],[18] This is despite the contradictory results described above and a recent review on macrolides in chronic inflammatory skin disorders stating that macrolides are best considered as experimental and should not be adopted into routine clinical practice until further studies are conducted and published. [19] The results of our study do not favor prescribing azithromycin for pityriasis rosea. It was found that the disease in both the groups improved over a period of time, which indeed reflects the natural course of the disease. However, the results of this trial should be confirmed by a larger multi-centric trial to exclude variations due to ethnicity or climatic conditions, if any and also by comparing different treatment durations.
Conclusion
Azithromycin does not affect the course of pityriasis rosea; neither the rash nor the associated pruritus. We would not recommend the use of this medication for the treatment of patients with pityriasis rosea. Good counseling regarding the natural, benign, self-limiting course of the disease and symptomatic treatment is all that may be required.
Acknowledgment
We would like to acknowledge the contribution of the Department of Biostatistics and Informatics, University College of Medical Sciences in performing the statistical evaluation.
| 1. |
Allen RA, Janniger CK, Schwartz RA. Pityriasis rosea. Cutis 1995;56:198-202.
[Google Scholar]
|
| 2. |
Cheong WK, Wong KS. An epidemiological study of pityriasis rosea in middle road hospital. Singapore Med J 1989;30:60-2.
[Google Scholar]
|
| 3. |
Chuh AA, Chiu SS, Peiris JS. Human herpesvirus 6 and 7 DNA in peripheral blood leucocytes and plasma in patients with pityriasis rosea by polymerase chain reaction: A prospective case control study. Acta Derm Venereol 2001;81:289-90.
[Google Scholar]
|
| 4. |
Amsden GW. Anti-inflammatory effects of macrolides - An underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother 2005;55:10-21.
[Google Scholar]
|
| 5. |
Scheinfeld NS, Tutrone WD, Torres O, Weinberg JM. Macrolides in dermatology. Clin Dermatol 2003;21:40-9.
[Google Scholar]
|
| 6. |
Sharma PK, Yadav TP, Gautam RK, Taneja N, Satyanarayana L. Erythromycin in pityriasis rosea: A double-blind, placebo-controlled clinical trial. J Am Acad Dermatol 2000;42:241-4.
[Google Scholar]
|
| 7. |
Chuh AA, Dofitas BL, Comisel GG, Reveiz L, Sharma V, Garner SE, et al. Interventions for pityriasis rosea. Cochrane Database Syst Rev 2007;2:CD005068. Available from: http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD005068/frame.html. [Cited on 2007 Apr 18].
[Google Scholar]
|
| 8. |
Amer A, Fischer H. Azithromycin does not cure pityriasis rosea. Pediatrics 2006;117:1702-5.
[Google Scholar]
|
| 9. |
Rasi A, Tajziehchi L, Savabi-Nasab S. Oral erythromycin is ineffective in the treatment of pityriasis rosea. J Drugs Dermatol 2008;7:35-8.
[Google Scholar]
|
| 10. |
Chuh AA. Diagnostic criteria for pityriasis rosea: A prospective case control study for assessment of validity. J Eur Acad Dermatol Venereol 2003;17:101-3.
[Google Scholar]
|
| 11. |
Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, et al. Assessment of pruritus intensity: Prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012;92:502-7.
[Google Scholar]
|
| 12. |
Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: Evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 2012;92:497-501.
[Google Scholar]
|
| 13. |
Leenutaphong V, Jiamton S. UVB phototherapy for pityriasis rosea: A bilateral comparison study. J Am Acad Dermatol 1995;33:996-9.
[Google Scholar]
|
| 14. |
González LM, Allen R, Janniger CK, Schwartz RA. Pityriasis rosea: An important papulosquamous disorder. Int J Dermatol 2005;44:757-64.
[Google Scholar]
|
| 15. |
Amer A, Fischer H, Li X. The natural history of pityriasis rosea in black American children: How correct is the "classic" description? Arch Pediatr Adolesc Med 2007;161:503-6.
[Google Scholar]
|
| 16. |
Chuang TY, Ilstrup DM, Perry HO, Kurland LT. Pityriasis rosea in Rochester, Minnesota, 1969 to 1978. J Am Acad Dermatol 1982;7:80-9.
[Google Scholar]
|
| 17. |
Singh V, Sharma M, Narang T, Madan M. Vesicular palmoplantar pityriasis rosea. Skinmed 2012;10:116-8.
[Google Scholar]
|
| 18. |
Ehsani A, Esmaily N, Noormohammadpour P, Toosi S, Hosseinpour A, Hosseini M, et al. The comparison between the efficacy of high dose acyclovir and erythromycin on the period and signs of pitiriasis rosea. Indian J Dermatol 2010;55:246-8.
[Google Scholar]
|
| 19. |
Alzolibani AA, Zedan K. Macrolides in chronic inflammatory skin disorders. Mediators Inflamm 2012;2012:159354.
[Google Scholar]
|
Fulltext Views
6,270
PDF downloads
3,359





