Translate this page into:
The frequency of osteoporosis in patients with pemphigus vulgaris on treatment
2 Department of Internal Medicine, Dicle University, Diyarbakir, Turkey
3 Department of Biophysics, Dicle University, Diyarbakir, Turkey
Correspondence Address:
Mehmet Harman
Department of Dermatology, Faculty of Medicine, Dicle �niversitesi Tip Fak�ltesi Deri ve Z�hrevi Hastaliklar Anabilim Dali, 21280 Diyarbakir
Turkey
| How to cite this article: U�mak D, Harman M, U�mak F, Akpolat V. The frequency of osteoporosis in patients with pemphigus vulgaris on treatment. Indian J Dermatol Venereol Leprol 2013;79:211-215 |
Abstract
Background: Pemphigus vulgaris was almost fatal before the advent of glucocorticoids. Unfortunately, the high doses and prolonged administration of glucocorticoids, which often needed to control the disease, result in numerous adverse effects many of which are serious. Aims: To evaluate the patients with pemphigus vulgaris on treatment in respect of osteoporosis and to compare the frequency of osteoporosis in these patients with the healthy ones. Methods: The study consisted of 40 patients with pemphigus vulgaris and 34 healthy controls. Bone mineral density measurements were obtained by dual- energy X-ray absorptiometry. Blood serum, bone parameters, and biochemical hormonal measurements were examined in both groups. Results: When the bone mineral density values of patients with pemphigus vulgaris were compared with those of the control group, there was no significant difference between hip bone mineral density values, while lumbar region T and Z scores were found significantly low in the patient group (p = 0.034 and p = 0.006, respectively). Osteoporosis, osteopenia, and normal dual-energy X-ray absorptiometry rates in the patient group were found to be 32.5%, 32.5%, and 35%, respectively. These rates were found to be 18%, 23%, and 59% in control group, respectively. There were more fractures in the patient group and the difference was statistically significant (p = 0.004). Conclusion: An increase in osteoporosis frequency and secondary fracture to osteoporosis in the patients with pemphigus vulgaris was detected.Introduction
Pemphigus vulgaris (PV) is an autoimmune blistering disease of the skin and mucous membranes with a high mortality if left untreated. The treatment of pemphigus is glucocorticoid (GC) centered as in the case of all other autoimmune diseases. [1] Although the systemic steroids form the basis of treatment in pemphigus, the utilization of these medications for long term in high doses might cause very serious adverse effects. [2] Osteoporosis is one of the major complications of steroid treatment. [3],[4] The patients with PV taking GC have a greater bone loss risk, because they have also other risk factors such as immobilization and malnutrition. The fractures in the spine and hips are related to increased morbidity and mortality. Prevention from glucocorticoid-induced osteoporosis (GIO) and acceptable treatment strategies have been published in many national journals and guides. [5],[6],[7],[8],[9] However, the number of publications related to the frequency of osteoporosis and its prevention in patients with PV is very few in dermatology journals.
In this prospective study, it was planned to evaluate the patients with PV receiving systemic steroid treatment in respect of osteoporosis and to compare the frequency of osteoporosis in these patients with those healthy ones.
Methods
Forty patients with PV that regularly attending the Department of Dermatology, Faculty of Medicine, Dicle University, Diyarbakýr, Turkey, from January 2, 2008 to March 13, 2009, and also 34 healthy individuals as the control group were studied prospectively. Age and sex matched healthy controls were selected from the spouses of the patients and the relatives of their spouses. Detailed medical histories of the group were recorded and all were examined physically. Demographical data was recorded, anthropometrical measures including weight, height and waist and hip circumferences were measured and body mass index (BMI), and waist to hip ratio (WHR) were calculated.
The patients were categorized into three subgroups according to the initial GC doses: <80 mg/day (mild disease), 80-120 mg/day (moderate disease), and >120 mg/day (severe disease). The initial dose was given for the first 2-4 weeks. Then we gradually tapered the steroid down until we reached a maintenance dose of 4 mg every other day.
We also classified patients according to the following GC exposure periods: Short (<6 months), medium (6 months to 5 years), and long (>5 years).
The history of other diseases (diabetes mellitus, hypertension, goiter, etc.), fractures and presence of osteoporosis in all cases and in their families, their life style, smoking and drinking, daily calcium intake and medications that might cause osteoporosis were recorded. In addition, dietary calcium intake [10] andphysical activity [11] were assessed and female cases were assessed in respect of menopause, premature menopause, and surgical menopause.
Full blood count, erythrocyte sedimentation rate, the biochemical tests as glucoses, creatinine, alanine amino transferase, albumin, alkaline phosphatase, calcium, phosphorous, and hormone tests as thyroid stimulating hormone, free T4, free T3, total T4, total T3, cortisol, and parathormone values were recorded. All of the patients and controls were questioned regarding the presence of thyroid diseases. The cases in whom hypo/hyperthyroid was detected were excluded from the study.
Bone mineral density (BMD; g/cm 2 ) was measured using Hologic Discovery QDR 4500A series technology with dual-energy X-ray absorptiometry (DEXA) method. Each patient was measured antero-posteriorly from lumbar region (L1-L4 vertebrae) and from femoral region (neck, trochanter major, intertrochanteric region, total and Ward′s triangle). The criteria of World Health Organization were taken as basis for the diagnosis of osteoporosis and osteopenia. According to T score values, values above -1 was considered normal, between -1 and -2.5 as osteopenia, and lower than -2.5 as osteoporosis.
SPSS 15.0 for Windows (SPSS Inc. Chicago, IL, USA) package program was used for statistical analysis. Mean and standard deviation values were used in the definitive statistics of continuous variables. Categorical variables were tested with cross tables and Chi-square analysis. Student′s t test was used in order to compare two independent group means for parametric variables. p < 0.05 were considered a significant difference.
The study was approved by the institutional ethic committee. Consent was obtained from the patients and healthy controls.
Results
The study was conducted with a patient group consisting of 40 individuals in total; 27 female (69%) and 13 male (32%) with PV diagnosed both clinically and pathologically, and also with a control group consisting of totally 34 healthy individuals; 25 female (74%) and 9 male (26%). Mean ages were 44.88±11.68 years and 48.97±15.06 years in patient and control groups, respectively.
The numbers of patients according to the initial GC doses <80 mg/day, 80-120 mg/day, and >120 mg/ day were 14, 21, and 2, respectively. The duration of GC taking ranged from 2 to 162 months. The median duration of steroid use was 30 months. The numbers of patients exposed to GC short, medium, and long period were 13, 20, and 7, respectively. All patients were receiving GC with oral calcium (600 mg daily) and vitamin D 3 (400 IU daily) supplementation. Only osteoporotic patients were taking antiresorptive therapy in addition to calcium and vitamin D 3 supplementation.
There was no statistically significant difference between the patient and control group in respect of age, gender ratio, BMI, WHR (p > 0.05). There was no difference between the patient and control group in respect of smoking and drinking.
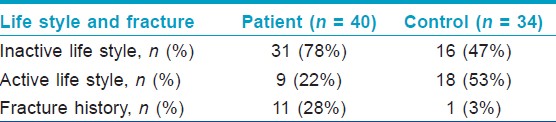
There was fracture history in 11 cases in the patient group and in 1 case in the control group. There was a significant difference between the patient and control group in respect of inactive life style and presence of fracture history in the Chi-square test (χ2 = 7.349, p = 0.007; χ2 = 8.159, p = 0.004, respectively) [Table - 1].
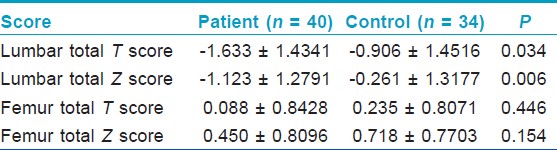
Total T and total Z scores of the lumbar and femur regions were evaluated in the bone densitometer with DEXA method in patient and control group. Accordingly, 13 patients with PV (32.5%) were evaluated as osteoporosis, 13 patients with PV as osteopenia (32.5%), and 14 patients with PV as normal (35%). These rates were found to be 18%, 23%, and 59% in control group, respectively. There was no statistical difference between the patient and control group in femur total T score and Z scores. However, the difference between the patient and control group in total T score and total Z scores of the lumbar region was statistically significant (p = 0.034, p = 0.006, respectively) [Table - 2].
Among three patient subgroups, categorized according to used corticosteroid doses, and duration of treatment, there was no statistical difference with respect to osteopenia and osteoporosis.
Discussion
Pemphigus, a common immunobullous disease of skin and mucous membranes, was almost fatal before the advent of corticosteroids. The mortality rate in pemphigus has been further reduced to 5% with the addition of adjuvant immunosuppressive medications. Nowadays, mortality and morbidity is generally dependent on treatment complications. [12],[13]
The effects of GC are well known; however, these have adverse effects and unfortunately these adverse effects are frequently observed. Many adverse effects are directly related to both dose and the treatment period. The one that can be foreseen and reduced the most among these adverse effects is bone loss that causes increase in osteoporosis, bone fracture risk in those that use it for long terms. [14] It has been stated that vertebral fractures might form in 30-50% of the patients that receive chronic GC treatment. [15] Baltzan [16] and Center [17] have shown in their study that osteoporosis might develop in one-third of those treated with GC in varying periods from 5 to 10 years.
Protection against osteoporosis is an important public health issue. Although there are no clear data for our country, the approximate national spending for osteoporosis and related fractures in 2002 in the USA has been estimated to be 18 billion dollars. [18]
In the update of the dermatology specific guide that Summey and Yosipovitch [19] prepared together; the postmenopausal female patients with an age of 65 and above, with light trauma fracture medical history, frequent falling medical history, or prednisone 20 mg/day and above have been defined as the high risk population.
BMD is reduced in the patients that use oral prednisolone in 5 mg or higher doses, however, it has been shown that BMD is reduced even in 2.5 mg/ day oral prednisolone doses. [20] American College of Rheumatology recommends the use of bisphosphonate in the event that daily 5 mg or higher dose prednisone is started for the cases whose long-term systemic corticosteroid treatment is planned. [21]
The trabecular loss in osteoporosis induced by GCs is more than the cortical loss. Thus, the first place where GIO is first detected is lumbar vertebrae. [22] The majority of these vertebral fractures are asymptomatic. [23] Bone loss can be determined with DEXA within a 6 month period and 5% of the bone mass is lost within the first year of the GC treatment. While bone loss is reduced further in the second and third years of the treatment, it continues especially in femur neck. [24]
BMD measurement was also used for determining the fracture risk in GIO. Although these measurements are accurate and correct, it has been put forward that it might show the fracture risk to be lower in corticosteroid areas. In the secondary osteoporosis that is formed due to medication use, the relation between the bone mass and bone durability is not a direct relation as in the case of primary osteoporosis. For example, 1 standard deviation reduction in BMD in postmenopausal women results in 2-fold increase in fracture risk. [25]
In a study by Van Staa et al.,[26] it has been shown that there is a relation between the oral GCs and the magnitude of fracture risk. When patients and control group are compared; the relative risk for the vertebral fracture of the patients with <2.5, 2.5-7.5, and >7.5 mg daily oral GC dose has been found as 1.6, 2.6, and 5.2, respectively. The relative risk for the hip fracture of these patients has been found as 1.0, 1.8, and 2.3. In another study by Van Staa et al.,[27] they evaluated the number of fractures related to systemic GC among the patients that applied to the general clinic and they detected oral GC in 47% of all hip fractures and in 72% of all vertebral fractures in the group that used systemic GC.
In the current study, the BMD reduction was detected in Lumbar T and Lumbar Z score DEXA measurements and this result complies with the literature. Although this low rate in BMD values has been found significant in patients with PV compared with the control group (p = 0.034 and p = 0.006, respectively). No correlation was found between the fractures and BMD values in patients with PV. This might be due to the fact that we detected fracture history with medical records information. Since, the fractures encountered in GIO, especially the ones in vertebral areas can be atraumatic and remain asymptomatic.
PV typically starts as painful erosions of the mucous lining of the mouth. Many patients may have nutritional deficits because of the pain associated with eating and swallowing, resulting in weight loss, fatigue, and malnutrition. Dehydration and abnormalities of electrolytes can occur as a result of nutritional deficits and extensive fluid loss from weeping of the ruptured bullae. Severe disease causes inactive lifestyle. High doses of corticosteroids (1-2 mg/kg daily) are widely used to control disease activity, with some regimens recommending up to 200-400 mg/d of prednisolone for severe disease. Because of these, PV patients have high risk for osteoporosis and fracture related to osteoporosis.
Wohl et al.,[28] identified a novel association between pemphigus and osteoporosis that was independent of GC use. The authors note that prior studies have reported osteoclast-mediated bone loss in other autoimmune and inflammatory conditions, including rheumatoid arthritis, lupus erythematosus, and chronic viral infection, suggesting a potential mechanism for the corticosteroid-independent risk of osteoporosis in pemphigus. They also found that the pemphigus-osteoporosis association is particularly strong in males and in patients less than 50 years of age.
There are some limitations in our study: (1) small sample size and limited statistical power, (2) some of our patients were immobile because of the active disease, and we could not exclude the possible negative effect of immobility on BMD, and (3) the absence of the quantitative computed tomography (QCT). Measurement of areal BMD by DEXA is the gold standard method for the assessment of bone fracture risk. However, DEXA measurements are 2-dimensional, and cannot differentiate between cortical and trabecular bone and is incapable of measuring these individual components. Newer technologies, such as QCT, measure only trabecular spinal bone density and would be a better technology for this purpose. [29]
In conclusion, osteoporosis frequency was found to be high among the pemphigus patients with treated systemic corticosteroid although they were advised to avoid themselves from osteoporosis and were supported with prophylactic calcium and vitamin D supplementation. Glucocorticoid-induced bone loss is the mostpredictable and debilitating complication of prolonged administrationof systemic GCs. In addition, malnutrition and inactive life style in pemphigus patients can contribute to osteoporosis. For these reasons, clinicians should consider screening, monitoring and providing preventive measures for osteoporosis in PV patients treated with systemic corticosteroid, and an antiresorptive treatment should be administered to the patients.
Acknowledgment
The authors wish to thank Yusuf Çelik, Professor, PhD, for providing assistance with statistical analyses.
| 1. |
Mimouni D, Anhalt GJ. Pemphigus. Dermatol Ther 2002;15:362-8.
[Google Scholar]
|
| 2. |
Bystryn JC, Steinman NM. The adjuvant therapy of pemphigus. An Update. Arch Dermatol 1996;132:203-12.
[Google Scholar]
|
| 3. |
Lamb A, Werth V. Osteoporosis in health and disease: A dermatologist′s perspective. Dermatol Clin2006;24:241-9.
[Google Scholar]
|
| 4. |
Williams LC, Nesbitt LT Jr. Update on systemic glucocorticosteroids in dermatology. Dermatol Clin 2001;19:63-77.
[Google Scholar]
|
| 5. |
Yood RA. Prevention of glucocorticoid-induced osteoporosis: Why are we doing so poorly? J Rheumatol 2006;33:1461-3.
[Google Scholar]
|
| 6. |
Sambrook PN. How to prevent steroid induced osteoporosis. Ann Rheum Dis 2005;64:176-8.
[Google Scholar]
|
| 7. |
Eastell R, Reid DM, Compston J, Cooper C, Fogelman I, Francis RM, et al. A UK Consensus Group on management of glucocorticoid-induced osteoporosis: An update. J Intern Med1998;244:271-92.
[Google Scholar]
|
| 8. |
Devogelaer JP, Goemare S, Boonen S, Body JJ, Kaufman JM, Reginster JY, et al. Evidence-based guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis: A consensus document of the Belgian Bone Club. Osteoporosis Int2006;17:8-19.
[Google Scholar]
|
| 9. |
McDonough RP, Doucette WR, Kumbera P, Klepser DG. An evaluation of managing and educating patients on the risk of glucocorticoid-induced osteoporosis. Value Health 2005;8:24-31.
[Google Scholar]
|
| 10. |
Angus RM, Sambrook PN, Pocock NA, Eisman JA. A simple method for assessing calcium intake in Caucasian women. J Am Diet Assoc 1989;89:209-14.
[Google Scholar]
|
| 11. |
Wicks J. Guide to exercise. Canberra: National Heart Foundation of Australia; 1983. p. 70-6.
[Google Scholar]
|
| 12. |
Turgutalp SÇ, Harman M. Pemfigusta Seyir ve Prognoz: 42 hastanýn deðerlendirilmesi. Dicle Týp Dergisi 2008;35:29-31.
[Google Scholar]
|
| 13. |
Yeh SW, Sami N, Ahmed RA. Treatment of pemphigus vulgaris: Current and emerging treatment options. Am J Clin Dermatol 2005;6:327-42.
[Google Scholar]
|
| 14. |
Canalis E. Mechanisms of glucocorticoid action in bone. Curr Osteoporos Rep 2005;3:98-102.
[Google Scholar]
|
| 15. |
Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporosis Int 2007;18:319-28.
[Google Scholar]
|
| 16. |
Baltzan MA, Suissa S, Bauer DC, Cummings SR. Hip fractures attributable to corticosteroid use. Study of Osteoporotic Fractures Group. Lancet 1999;353:1327.
[Google Scholar]
|
| 17. |
Center JR, Nguyen TV, Schneider D, Sambrook P, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: An observational study.Lancet 1999;353:878-82.
[Google Scholar]
|
| 18. |
National Osteoporosis Foundation (Internet). Osteoporosis: A debilitating disease that can be prevented and treated. Available from: http://www.nof.org. [Last accessed on 2012 May 9].
[Google Scholar]
|
| 19. |
Summey BT, Yosipovitch G. Glucocorticoid-induced bone loss in dermatologic patients: An Update. Arch Dermatol2006;142:82-90.
[Google Scholar]
|
| 20. |
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res2000;15:993-1000.
[Google Scholar]
|
| 21. |
American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. Arthritis Rheum 2001;44:1496-503.
[Google Scholar]
|
| 22. |
Vermaat H, Kirtschig G. Prevention and treatment of glucocorticoid-induced osteoporosis in daily dermatologic practice.Int J Dermatol 2008;47:737-42.
[Google Scholar]
|
| 23. |
Angeli A, Guglielmi G, Dovio A, Capelli G, de Feo D, Giannini S, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: A cross-sectional outpatient study. Bone 2006;39:253-9.
[Google Scholar]
|
| 24. |
Adachi JD, Papaioannou A. Corticosteroid-induced osteoporosis: Detection and management. Drug Saf2001;24:607-24.
[Google Scholar]
|
| 25. |
Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254-9.
[Google Scholar]
|
| 26. |
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: Relationship to daily and cumulative doses. Rheumatology2000;39:1383-9.
[Google Scholar]
|
| 27. |
Van Staa TP, Abenhaim L, Cooper C, Zhang B, Leufkens HG. Public health impact of adverse bone effects of oral corticosteroids. Br J Clin Pharmacol 2001;51:601-7.
[Google Scholar]
|
| 28. |
Wohl Y, Dreiher J, Cohen AD. Pemphigus and Osteoporosis: Case-control study. Arch Dermatol 2010;146:1126-31.
[Google Scholar]
|
| 29. |
Chiodini I, Francucci CM, Scillitani A. Densitometry in glucocorticoid-induced osteoporosis. J Endocrinol Invest 2008;31:33-7.
[Google Scholar]
|
Fulltext Views
3,814
PDF downloads
1,634





