Translate this page into:
The nail as an investigative tool in medicine: What a dermatologist ought to know
2 Department of Dermatology and STD, VMMC and Safdarjung Hospital, New Delhi, India
Correspondence Address:
Chander Grover
Department of Dermatology and STD, UCMS and GTB Hospital, Dilshad Garden, Delhi - 110 095
India
| How to cite this article: Grover C, Bansal S. The nail as an investigative tool in medicine: What a dermatologist ought to know. Indian J Dermatol Venereol Leprol 2017;83:635-643 |
Abstract
The nail is an important skin appendage, but not many dermatologists are aware of the importance it receives outside our specialty. This article focuses on the nail in non-dermatological contexts. The nail is a keratinized matrix capable of continuous growth with the ability to incorporate various compounds within its structure. Therefore it can be used to monitor long-term consumption of drugs. It is also an excellent source of germ-line DNA for genetic analyses. With an increased undrstanding of nail physiology, there is now a better understanding of its connection to various pathologies as well. Nails, being peripherally placed, are easy to sample without significant discomfort to the patient, making them a valuable diagnostic tool. For this narrative review, we carried out a PubMed search using the key words “nail clipping,” “nail DNA,” “nail diabetes mellitus;” “nail clipping oncology,” and “nail forensics”. Retrieved articles were searched for information pertaining to non-dermatologic uses of nail for evaluation, which is presented in a narrative fashion. It is clear from recent literature that the nail is not just an inert skin appendage, but a dynamic window into the ever-changing metabolic and genetic milieu. We highlight the numerous roles of nail specimens, as well as point towards future research needed therein.Introduction
Over the years, there is increasing interest in the study of the nail in health and disease. We know of its special structure and biological uses. It is also useful in diagnosis and as a marker of systemic disease. However, not many dermatologists know the amount of attention it receives outside our specialty.
This article focuses on the non dermatological relevance of this appendage. With developments in molecular biology and genetics, the nail is increasingly being seen as an ideal source of obtaining human specimens. It has attained the status of “a true window”, not just to disease, but also to the health status of an individual.
Methods and Results
For this review, information was collected by a PubMed search of articles published regarding the nondermatological uses of nail specimens. We used the key-words “nail clipping,” “nail DNA,” “nail diabetes mellitus,” “nail clipping oncology,” “nail forensic,” and “nail biometrics.” The searches yielded 82, 685, 437, 8, 122, and 2 indexed articles respectively, in English. These articles were retrieved and classified as case reports, review articles, and clinical trials. Information pertaining to nondermatologic applications of nails was collected. The final data was analyzed and is presented in a narrative fashion.
Why Use the Nail?
With the increasing popularity of screening programs, the need for appropriate human tissue specimens has increased. The specimen should afford adequate sensitivity and specificity in detecting what it is supposed to detect; it should involve low costs, collection should cause minimal discomfort to both patients and practitioners, and it should be easy to store and transport.[1] The nail satisfies most of these criteria.
Conventionally, human blood and serum are commonly used for diagnosis; however, the importance of alternative tissue sources has increased over the years due to various reasons. Venous blood collection may prove difficult, especially in special populations, for large-scale programs, or for international collaborative investigations.[2] Alternative tissue sources include card-based blood spots, buccal scrapes, hair samples and nail clippings as these are uniquely accessible as well as capable of delivering host DNA and other details. Nail as an alternative tissue sources has been found useful for genetic diagnosis as a part of screening procedures,[3] diagnostic procedures,[4],[5] assessment of adverse reactions,[6] familial and population genetic profiling,[7] and molecular autopsy studies.[8],[9] In fact, for molecular autopsy studies, nail may be the only specimen which can be used for defining the cause of death or for clinical genetic information important for the surviving family.[2] In addition to the advantages of adequate sensitivity and specificity, low cost, ease of retrieval, minimal discomfort upon retrieval, and acceptability to both patients and practitioners, nail specimens are also easy to collect, store, and transport.[1]
However, because of a lack of awareness and proper processing techniques in routine laboratories, nail samples have not widely been used as diagnostic tools.
Nail as a keratinized matrix
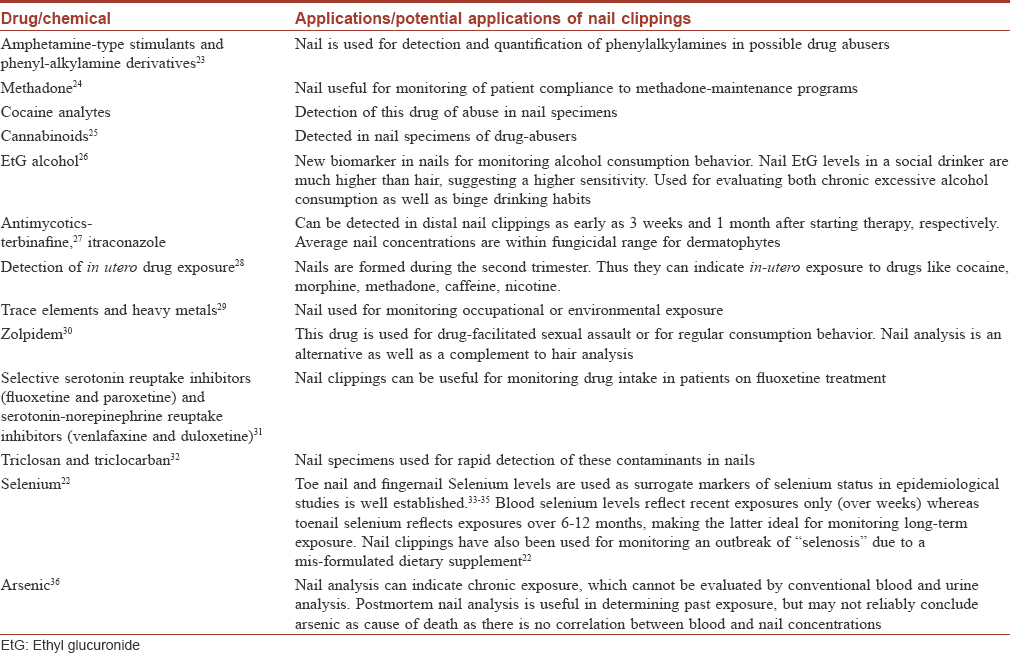
Both nail and hair are keratinized matrices capable of continuous growth, and incorporate compounds within their structure. This property can be utilized in monitoring long-term consumption of alcohol or drugs.[10] Nail specimens are found useful in toxicology and especially as an alternative to hair specimens.[10] Hair analysis has been an established tool for drug testing, driving ability examination, detection of gestational drug exposure, criminal assault, and post-mortem toxicology.[11] Correspondingly, our understanding of the mechanisms of incorporation of drugs into the hair matrix is advanced.[12],[13] In contrast, literature on incorporation mechanisms in nails is sparse;[11] nevertheless, we know the following mechanisms of drug incorporation in nails:[1]
- Nail matrix incorporation occurs during the formation of the nail plate via matrix blood flow. Thus, an incorporated drug would be detectable only when the nail grows enough to reach the free edge (10–18 weeks based on average nail growth rate)
- Nail bed incorporation occurs during nail thickening. The nail bed contributes 21% of the nail thickness.[14] A drug incorporated in this manner would be detectable in distal nail clippings much earlier (as early as 2–3 weeks)[15],[16],[17]
- Environmental contamination occurs mostly in the distal part, which explains the presence of materials, mostly xenobiotics, in the distal nail [18]
- Sweat-mediated transport is responsible for rapid initial incorporation (within 24 hours) of drugs such as zolpidem.[1] Drugs are eliminated through sweat channels depending on their molecular weight and hydrophilicity.[19] Nails have a water content of 9–10% and drug diffusion through the nail bed can lead to early detection at the free edge.[14],[20] Laufen et al. reported an uptake of fluconazole as early as 8 hours after intake.[17] Similar diffusion for topically applied terbinafine has also been studied.[21]
The kinetics of drug incorporation in the nails have been especially well worked out for zolpidem, a drug used for drug-facilitated sexual assault.[10] A single dose administered has been found to be detectable in all fingernail clippings from as early as 24 h to as late as 3.5 months. In fact, even the time of intake can be inferred from the analysis of single fingernail clippings. Nail analysis could thus be an alternative as well as a complement to hair analysis in cases of suspected drug-facilitated sexual assault, and for monitoring of consumption behaviour.
[Table - 1] summarizes drugs routinely and reliably tested for, in nail specimens. This “nail biologic monitor” has been found useful in monitoring long-term exposure to drugs, micronutrients and xenobiotics; it can even help in temporal correlation with the supposed period of exposure.[22]
Nail as a source of DNA
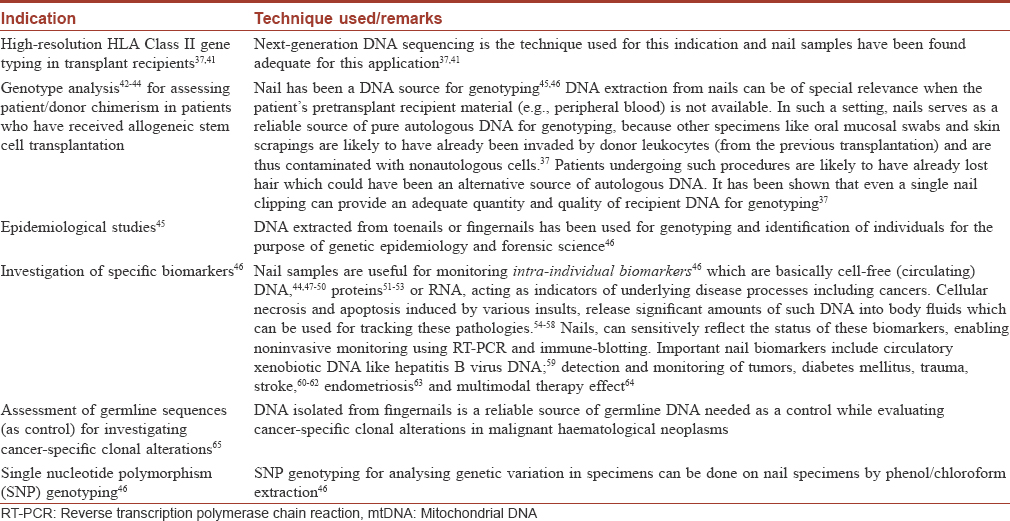
Fingernail material is an excellent source of germline DNA for genetic analyses in almost all clinical settings.[37] Although the use of hair for this indication is well-known, there are practical problems, with hair specimens often being reported inferior for diagnostic use due to poorly detectable DNA. The Baylor SUDEP Tissue Donation Program (STOP) reported that hair samples were often received without follicles or that their integrity may be compromised by prior chemical processing with hair-care products.[2] Fingernails are a more reliable source of autologous DNA of high-quality.[37]
The specialized structure of fingernails (embodying DNA in keratinized cells) makes DNA extraction more complex than with fresh somatic cells; hence, well-defined protocols and reagents have been designed for lysing keratin.[38] These protocols optimize the yield and quality of pure, intact DNA which has been found good enough even for demanding techniques such as next-generation sequencing for HLA typing.[37] Advanced techniques such as the Prepfiler Forensic DNA Extraction kit can yield a mean of 1 mg high-quality DNA (range, 0.5 to 2.3 mg) from 20 mg nail material (1 to 10 pieces of fingernail clippings, a few millimetres wide only).[37] DNA extracted from toenails [39] or fingernails [40] has been used for genotyping and identification of individuals in genetic epidemiology and forensic studies. Some of the indications for nail plate-derived DNA are summarized in [Table - 2].
How to Collect Nail Specimens
Collection of nail specimens is as simple as collection of fingernail trimmings or overhang of nail plates.[2] Specimens can be collected on a plain sheet of paper (as done for mycology) or in sterile 1.5 mL microcentrifuge tubes for more demanding DNA analyses. An adequate sample consists of at least 1 week of untampered fingernail growth (assuming an average growth rate of 3 mm/month).[2],[66] Except for daily hygiene, no additional nail cosmetic or nail treatment should have been done. Hands are thoroughly washed with soap and warm water and allowed to dry. Sterilized conventional metal nail clippers are used and whole nail trimmings are transferred into pre-labelled tubes or containers for transportation. These can easily be stored at room temperature until use. Depending on the analyses required, only one nail clipping may be collected (for serial detection of drugs)[10] or ten nails (for DNA analyses) may be collected. For serial detection of drugs, ring fingernails are preferred (because of their medium growth rate) and serial collection from the same nail is advised.[67]
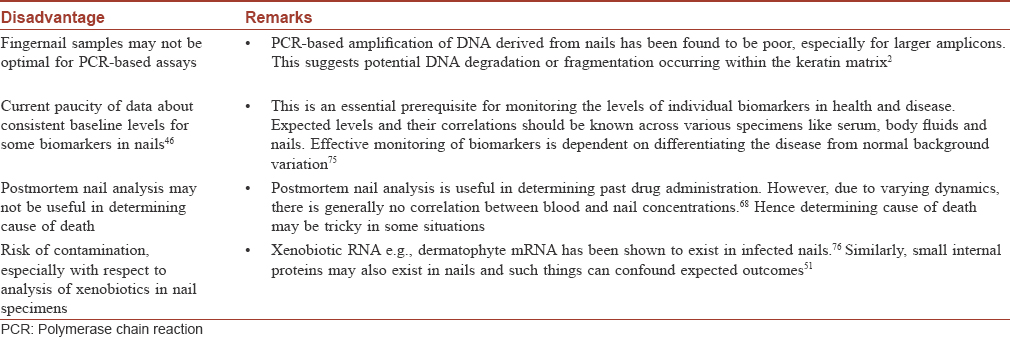
[Table - 3] and [Table - 4] summarize the advantages and disadvantages of using nails as a specimen.

Techniques Used to Examine Nail
As the nail has a unique structure, specialized techniques are required to examine and quantify specific components. Some such techniques used for nail analysis are summarized below.
- Laser-induced breakdown spectroscopy is used for the analysis of varied biological substrates such as bacteria,[77],[78] teeth,[29],[79] hair,[80] bones [81] and fingernails.[82] It employs a focused high-power, short-pulsed laser beam directed onto the nail surface.[1] Based on the analysis of emission spectra from the surface, varying elements can be analyzed.
- High-performance liquid chromatography has been used for determination of drugs such as selective serotonin-reuptake inhibitors and serotonin–norepinephrine reuptake inhibitors in nail clippings.[68] It has also been used as a speedy, simple and accurate technique in forensic toxicology for elucidating the cause of death or drug abuse
- Ultraperformance liquid chromatography–tandem mass spectrometry has been used to detect triclosan and triclocarban in nails.[83] The collected nail clippings are digested with sodium hydroxide and chromatographic separation is performed with methanol. Target compounds are then determined by mass spectrometry
- Micro-PIXE (particle-induced X-ray emission) and micro-RBS (Rutherford back-scattering spectrometry) have been used to determine three-dimensional concentration maps of 18 elements in the human nail viz., major elements (C, N, and O), minor elements (P, S, Cl, K, and Ca), and trace elements (Fe, Mn, Zn, Ti, Na, Mg, Rb, Br, Sr, and Se)[84]
- Hard X-ray micro-analysis has been used to examine arsenic distribution in nail-clippings.[36] Nail clippings embedded in polyester resin and cut in cross-sectional slices are analyzed for arsenic concentration in different areas
- Synchrotron-based XRF (X-ray fluorescence) mapping has also been used to evaluate arsenic micro-distribution in toenail clippings.[2],[85]
Clinical Indications for Use of Nail Specimens
The diagnostic use of nail specimens is well established for the following indications:
Nail in diabetes mellitus
Diabetes mellitus is a metabolic disease characterized by high blood sugar either due to insufficient insulin production or poor responsiveness.[86] Various systemic pathologic alterations and metabolic events in diabetes mellitus are known to affect the nail unit structure and composition, and a nail sample can be a useful for clinical investigations.[87],[88],[89] Documented techniques for the detection and monitoring of diabetes in the nail unit include:
- Estimation of glycated nail proteins has been found to reflect average blood glucose control over the previous 6–9 months.[90],[91] An analysis is possible even on a 10 mg sample. The normal reference range for glycated nail protein is 0.55–3.60 μmol/g nail. In diabetics, the values are significantly higher (median, 4.07 μmol/g nail). Nail analysis could therefore be a simple alternative for diagnosing diabetes in persons from remote areas
- Changes in the molecular structure of human fingernail proteins in diabetic and nondiabetic specimens have been documented on the basis of their Fourier transform infrared spectroscopy spectra.[91] It has been concluded that nail proteins of diabetics contain α-helical structure (including the presence of amide II bonds), whereas nails of nondiabetic patients do not have the amide II structures
- The dielectric properties of keratin–water system in diabetic and healthy fingernails have also been evaluated.[92] It was reported that the dielectric measurements of human nail could be used for the detection of diabetes
- Nasli-Esfahani et al. carried out elemental analysis of nail as well as other biological samples (serum, scalp hair, urine and other body fluids) in diabetic patients.[93] They concluded that scalp hair and nail are the best biological samples for trace element analysis in diabetics (especially Cr, Se, and Mn)
- Laser-induced breakdown spectroscopy (LIBS) has been used for analyzing diabetic and nondiabetic nails.[1] It was concluded that LIBS spectra of fingernail can be a valid screening tool for diabetics in a large population with the advantages of quick measurement, broad elemental coverage, and low cost [1]
- Nail fold capillaroscopic (NFC) images were evaluated quantitatively as well as qualitatively in 145 children with Type 1 diabetes mellitus.[90] Increases in the number as well as length of capillaries, presence of mega-capillaries and Raynaud's loops, and intense red background (possible neoangiogenesis) were the recorded findings. Longer disease duration correlated with an increase in the number of capillaries, disturbances in distribution, as well as the presence of abnormal capillaries. NFC is a noninvasive, painless and easily repeatable test allowing digital storage of images; it can be used as an effective monitoring tool in diabetics.[90]
Role of nail clippings in oncology
Recent oncological research has focused on the primary prevention of cancer and identifying individuals at risk. It is known that trace elements have inhibitory as well as causative roles in oncogenesis. In addition, exposure to trace elements can be predetermined (e.g. arsenic) and may also be a modifiable risk-factor.[91] Nail-clippings are therefore regarded as valuable biomarkers for such exposures.[93],[94]
The FINBAR (Factors Influencing Barrett's Adenocarcinoma Relationship) study group attempted to correlate trace element status in toenails with the risk of Barrett's oesophagus and oesophageal adenocarcinoma.[91] Toenail clippings from 638 participants and healthy controls were analyzed for eight trace elements. There was a two-fold higher risk of Barrett's oesophagus with high toenail zinc, a borderline significant, increased risk with higher cobalt levels, and no association with levels of chromium, cerium, mercury, and selenium.
The nail has been proposed as a more reliable biological meter for arsenic than serum because elevated levels would be maintained in the former for a longer time. Further, external contamination of nails with arsenic is much less extensive compared to that of hair.[94] Long-term exposure to arsenic can lead to adverse health effects [95],[96] Lu et al. concluded that high arsenic exposure in humans promotes cancer initiation,[97] though the exact mechanism of arsenic's role in carcinogenesis remains unknown.[98],[99] Mechanisms responsible for arsenic accumulation in nails are poorly understood. The affinity of arsenic to sulphydryl groups of nail keratins may be responsible.[100]
Forensic importance of nails
The nail plate is an important substrate for diagnosis in forensic science.[101] Forensic casework routinely involves examination of fingernail scrapings and clippings for foreign DNA. In this scenario, both in-vivo and in-vitro analysis of nail specimens assumes significance. Though finger-nails may not be as useful as fingerprints for identification, in many cases broken fingernail plates have been used to associate a suspect with the victim by comparing nail ridge patterns.[102]
Matte et al. reported that up to 19% of the general population may have foreign DNA beneath their fingernails, whereas foreign DNA may be detected in 33% of forensic fingernail samples.[103] The normally present foreign DNA also tends not to persist for long. This needs to be taken into account by forensic analysts when providing an opinion on the relevance of foreign DNA under fingernails.
In forensic toxicology, reports abound on the usefulness of detecting drugs of abuse in nails. Brown et al. reported the utility of fingernail clippings in testing for levels of anabolic steroids in sportspersons with doping charges.[104] Other reports include amphetamine-type stimulants, methadone, cocaine breakdown products, phenylalkylamine derivatives, and cannabinoids being detected.[105],[106],[107],[108],[109],[110],[111],[112],[113] Further, ethyl glucuronide (EtG) has been put forward as a new biomarker in nails for alcohol consumption behavior.[114]
Miscellaneous medical disorders
Apart from the diseases discussed above, nail clippings/biopsies have been useful in other diseases. The utility of the “nail window” into systemic diseases cannot be undermined.[115]
Nail biopsies have been found useful in gout to detect urate crystals in subungual horn.[116] Tirado-González et al. described subungual urate crystals extruded subclinically in some cases of gout.[116] Nail biopsies taken to evaluate fungal elements showed urate crystals instead and the history subsequently confirmed gout. It was reported that there were no tophi noted in or near the nail field. Such crystals probably occur via exudation/transudation of fluids into the nail structure, offering a “nail window” into haematic or metabolic abnormalities. The authors concluded that the cytological and histological findings in nail specimens could be used to evaluate nail diseases as well as systemic diseases.[116]
Similarly, toenail nicotine levels have been used as biomarkers to predict the risk of coronary heart disease (CHD). In a nested case-control study involving 62,641 women followed up over 16 years,[117] a statistically significant, dose-response association was seen between increased toenail nicotine levels and risk of CHD. The authors concluded that toenail nicotine levels are predictive of CHD among women independent of other risk factors.
Nails for biometrics
With advances in information technology, security aspects have become paramount. Authentication is a prerequisite for security with biometric authentication being an important mode. This involves automated recognition of individuals based on their physiological characteristics, identifying a person based on “who she/he is” rather than “what she/he has” (card, token, key); or “what she/he knows” (password, pin).[119] Common characteristics used for biometrics include face recognition, fingerprints, handwriting, hand geometry, iris, vein, voice or retinal scan. The use of fingernail patterns as biometric markers has been evaluated and found to be useful.[118] Herein, authentication is based on unique individual ridge patterns of the nail bed reflected on the nail plate surface, which can be evaluated by computational analysis even in low-resolution images.[120] This has proved to be a very unique and stable biometric identifier, good enough for forensic as well as civilian applications.[120] Extensive experimentation has validated its use.
The low-resolution nail plate images are acquired with a contactless, unconstrained imaging setup analyzing texture-based feature descriptors. Then, computational analysis is used for integrating nail plates from three fingers. Outcomes of rigorous experimental analysis on 2700 nail plate images found this to be a promising biometric modality.[121] Nail bed pattern can also be analyzed with a laser-based broadband interferometer technique.[122] Another system measuring spacing of the capillary loops with highly monochromatic light has also been evolved.[123]
Hand-based biometrics has high user acceptance and reliability. Of these, the fingernail plate is characterized by high individuality,[124],[125] with a high degree of distinctiveness, even among identical twins.[126] Moreover, the hardened nail resists environmental effects, barring changes caused by nail diseases/disorders and malnutrition.[127] This ensures high reproducibility as well.
Radiation dosimetry
Rapid and accurate determination of individual radiation exposure would be needed to screen exposed populations in case of a radiological/nuclear event. It has been seen that estimating the chemical or physical alterations produced in biomaterials can be used to determine the level of exposure. Radiation sensitivity of nails is relatively high and changes produced in nails exposed to radiation have been found to be a useful biodosimetry method. In addition, the radicals generated in condensed nail protein are stable over time.[128],[129] An ex-vivo estimation of severity of radiation exposure based on the electron paramagnetic resonance nail dosimetry was evaluated by He et al.[128] Human nail clippings were used to evaluate stable radiation-induced signal. It was found that a reliable triage based on radiation dosage was possible.[130] The technique also ensured immediate and rapid dose assessment.
This review has some limitations. The topic being vast, we may have missed additional diagnostic applications of the nail unit not adequately represented in the indexed literature. In addition, with expanding developments in the field, a compendium such as this may fall short of the latest information at times despite our efforts to make it up-to-date.
Conclusion
It is clear from the recent growth in literature that the nail is not just an inert skin appendage, but. a dynamic part of the human body, reflective of the changes in the metabolic and genetic milieu. Nail specimens are a valuable diagnostic tool as they are easy to retrieve, without causing significant discomfort. The coming years are likely to see more research and expansion of knowledge in this field.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Bahreini M, Ashrafkhani B, Tavassoli SH. Discrimination of patients with diabetes mellitus and healthy subjects based on laser-induced breakdown spectroscopy of their fingernails. J Biomed Opt 2013; 18:107006.
[Google Scholar]
|
| 2. |
Klassen TL, von Rüden EL, Drabek J, Noebels JL, Goldman AM. Comparative analytical utility of DNA derived from alternative human specimens for molecular autopsy and diagnostics. J Mol Diagn 2012; 14:451-7.
[Google Scholar]
|
| 3. |
Gong IY, Tirona RG, Schwarz UI, Crown N, Dresser GK, Larue S, et al. Prospective evaluation of a pharmacogenetics-guided warfarin loading and maintenance dose regimen for initiation of therapy. Blood 2011; 118:3163-71.
[Google Scholar]
|
| 4. |
Kapplinger JD, Tester DJ, Salisbury BA, Carr JL, Harris-Kerr C, Pollevick GD, et al. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm 2009; 6:1297-303.
[Google Scholar]
|
| 5. |
Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm 2005; 2:507-17.
[Google Scholar]
|
| 6. |
Pirmohamed M. Acceptance of biomarker-based tests for application in clinical practice: Criteria and obstacles. Clin Pharmacol Ther 2010; 88:862-6.
[Google Scholar]
|
| 7. |
Klassen T, Davis C, Goldman A, Burgess D, Chen T, Wheeler D, et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell 2011; 145:1036-48.
[Google Scholar]
|
| 8. |
Johnson JN, Tester DJ, Bass NE, Ackerman MJ. Cardiac channel molecular autopsy for sudden unexpected death in epilepsy. J Child Neurol 2010; 25:916-21.
[Google Scholar]
|
| 9. |
Skinner JR, Crawford J, Smith W, Aitken A, Heaven D, Evans CA, et al. Prospective, population-based long QT molecular autopsy study of postmortem negative sudden death in 1 to 40 year olds. Heart Rhythm 2011; 8:412-9.
[Google Scholar]
|
| 10. |
Madry MM, Steuer AE, Binz TM, Baumgartner MR, Kraemer T. Systematic investigation of the incorporation mechanisms of zolpidem in fingernails. Drug Test Anal 2014; 6:533-41.
[Google Scholar]
|
| 11. |
Pragst F, Balikova MA. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta 2006; 370:17-49.
[Google Scholar]
|
| 12. |
Henderson GL. Mechanisms of drug incorporation into hair. Forensic Sci Int 1993; 63:19-29.
[Google Scholar]
|
| 13. |
Cone EJ. Mechanisms of drug incorporation into hair. Ther Drug Monit 1996; 18:438-43.
[Google Scholar]
|
| 14. |
Johnson M, Shuster S. Continuous formation of nail along the bed. Br J Dermatol 1993; 128:277-80.
[Google Scholar]
|
| 15. |
Finlay AY. Pharmacokinetics of terbinafine in the nail. Br J Dermatol 1992; 126 Suppl 39:28-32.
[Google Scholar]
|
| 16. |
Matthieu L, De Doncker P, Cauwenbergh G, Woestenborghs R, van de Velde V, Janssen PA, et al. Itraconazole penetrates the nail via the nail matrix and the nail bed – an investigation in onychomycosis. Clin Exp Dermatol 1991;16:374-6.
[Google Scholar]
|
| 17. |
Laufen H, Zimmermann T, Yeates RA, Schumacher T, Wildfeuer A. The uptake of fluconazole in finger and toe nails. Int J Clin Pharmacol Ther 1999;37:352-60.
[Google Scholar]
|
| 18. |
Goullé JP, Saussereau E, Mahieu L, Bouige D, Groenwont S, Guerbet M, et al. Application of inductively coupled plasma mass spectrometry multielement analysis in fingernail and toenail as a biomarker of metal exposure. J Anal Toxicol 2009;33:92-8.
[Google Scholar]
|
| 19. |
De Giovanni N, Fucci N. The current status of sweat testing for drugs of abuse: A review. Curr Med Chem 2013;20:545-61.
[Google Scholar]
|
| 20. |
Lorincz AL, Stoughton RB. Specific metabolic processes of skin. Physiol Rev 1958;38:481-502.
[Google Scholar]
|
| 21. |
Munro CS, Shuster S. The route of rapid access of drugs to the distal nail plate. Acta Derm Venereol 1992;72:387-8.
[Google Scholar]
|
| 22. |
Morris JS, Crane SB. Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients 2013;5:1024-57.
[Google Scholar]
|
| 23. |
Kim JY, Cheong JC, Lee JI, Son JH, In MK. Rapid and simple GC-MS method for determination of psychotropic phenylalkylamine derivatives in nails using micro-pulverized extraction. J Forensic Sci 2012;57:228-33.
[Google Scholar]
|
| 24. |
Lemos NP, Anderson RA, Robertson JR. The analysis of methadone in nail clippings from patients in a methadone-maintenance program. J Anal Toxicol 2000;24:656-60.
[Google Scholar]
|
| 25. |
Kim JY, Cheong JC, Kim MK, Lee JI, In MK. Simultaneous determination of amphetamine-type stimulants and cannabinoids in fingernails by gas chromatography-mass spectrometry. Arch Pharm Res 2008;31:805-13.
[Google Scholar]
|
| 26. |
Morini L, Colucci M, Ruberto MG, Groppi A. Determination of ethyl glucuronide in nails by liquid chromatography tandem mass spectrometry as a potential new biomarker for chronic alcohol abuse and binge drinking behavior. Anal Bioanal Chem 2012;402:1865-70.
[Google Scholar]
|
| 27. |
Finlay AY. Pharmacokinetics of terbinafine in the nail. Br J Dermatol 1992;39 126 Suppl:28.
[Google Scholar]
|
| 28. |
Mari F, Politi L, Bertol E. Nails of newborns in monitoring drug exposure during pregnancy. Forensic Sci Int 2008;179:176-80.
[Google Scholar]
|
| 29. |
Samek O, Beddows DC, Telle HH, Morris GW, Liska M, Kaiser J. Quantitative analysis of trace metal accumulation in teeth using laser-induced breakdown spectroscopy. Appl Phys A 1999;69:S179-82.
[Google Scholar]
|
| 30. |
Carter LP. Potential impact of drug effects, availability, pharmacokinetics, and screening on estimates of drugs implicated in cases of assault. Drug Test Anal 2011;3:586-93.
[Google Scholar]
|
| 31. |
Samanidou V, Pantazidou K, Kovatsi L, Njau S, Livanos A. A simple HPLC method for the simultaneous determination of two selective serotonin reuptake inhibitors and two serotonin-norepinephrine reuptake inhibitors in hair, nail clippings, and cerebrospinal fluid. J Sep Sci 2012;35:839.
[Google Scholar]
|
| 32. |
Shi Y, Zhang J, Lu L, Shao B. Determination of triclosan and triclocarban in human nails by solid-phase extraction and ultra-performance liquid chromatography-tandem mass spectrometry. Se Pu 2013;31:1040.
[Google Scholar]
|
| 33. |
Longnecker MP, Taylor PR, Levander OA, Howe M, Veillon C, McAdam PA, et al. Selenium in diet, blood, and toenails in relation to human health in a seleniferous area. Am J Clin Nutr 1991;53:1288-94.
[Google Scholar]
|
| 34. |
Steven Morris J, Stampfer MJ, Willett W. Dietary selenium in humans toenails as an indicator. Biol Trace Elem Res 1983;5:529-37.
[Google Scholar]
|
| 35. |
Morris JS, Ngwenyama R, Guthrie JM, Brockman JD, Spate VL, Robertson JD. Quality control in the neutron activation analysis of biological markers for selenium in epidemiological investigations. J Radioanal Nucl Chem 2008;276:7-13.
[Google Scholar]
|
| 36. |
Gherase MR, Desouza ED, Farquharson MJ, McNeill FE, Kim CY, Fleming DE, et al. X-ray fluorescence measurements of arsenic micro-distribution in human nail clippings using synchrotron radiation. Physiol Meas 2013;34:1163-77.
[Google Scholar]
|
| 37. |
Preuner S, Danzer M, Pröll J, Pötschger U, Lawitschka A, Gabriel C, et al. High-quality DNA from fingernails for genetic analysis. J Mol Diagn 2014;16:459-66.
[Google Scholar]
|
| 38. |
Schraml E, Daxberger H, Watzinger F, Lion T. Quantitative analysis of chimerism after allogeneic stem cell transplantation by PCR amplification of microsatellite markers and capillary electrophoresis with fluorescence detection: The Vienna experience. Leukemia 2003;17:224-7.
[Google Scholar]
|
| 39. |
Jones J, Jones M, Plate C, Lewis D, Fendrich M, Berger L, et al. Liquid chromatography-tandem mass spectrometry assay to detect ethyl glucuronide in human fingernail: Comparison to hair and gender differences. Am J Analyt Chem 2012;3:83-91.
[Google Scholar]
|
| 40. |
Skopp G, Pötsch L. A case report on drug screening of nail clippings to detect prenatal drug exposure. Ther Drug Monit 1997;19:386-9.
[Google Scholar]
|
| 41. |
Danzer M, Niklas N, Stabentheiner S, Hofer K, Pröll J, Stückler C, et al. Rapid, scalable and highly automated HLA genotyping using next-generation sequencing: A transition from research to diagnostics. BMC Genomics 2013;14:221.
[Google Scholar]
|
| 42. |
Tanigawara Y, Kita T, Hirono M, Sakaeda T, Komada F, Okumura K. Identification of N-acetyltransferase 2 and CYP2C19 genotypes for hair, buccal cell swabs, or fingernails compared with blood. Ther Drug Monit 2001;23:341-6.
[Google Scholar]
|
| 43. |
Matsuzawa N, Shimozato K, Natsume N, Niikawa N, Yoshiura K. A novel missense mutation in van der woude syndrome: Usefulness of fingernail DNA for genetic analysis. J Dent Res 2006;85:1143-6.
[Google Scholar]
|
| 44. |
van Breda SG, Hogervorst JG, Schouten LJ, Knaapen AM, van Delft JH, Goldbohm RA, et al. Toenails: An easily accessible and long-term stable source of DNA for genetic analyses in large-scale epidemiological studies. Clin Chem 2007;53:1168-70.
[Google Scholar]
|
| 45. |
Park J, Liang D, Kim JW, Luo Y, Huang T, Kim SY, et al. Nail DNA and possible biomarkers: A pilot study. J Prev Med Public Health 2012;45:235-43.
[Google Scholar]
|
| 46. |
Lecomte T, Berger A, Zinzindohoué F, Micard S, Landi B, Blons H, et al. Detection of free-circulating tumor-associated DNA in plasma of colorectal cancer patients and its association with prognosis. Int J Cancer 2002;100:542-8.
[Google Scholar]
|
| 47. |
Nie SJ, Yang YM, Tang WR, Xu BY, Jing Q, Xiao CJ, et al. Extraction and analysis of nuclear DNA from free margin of nail material. Yi Chuan 2007;29:1373-7.
[Google Scholar]
|
| 48. |
Anderson TD, Ross JP, Roby RK, Lee DA, Holland MM. A validation study for the extraction and analysis of DNA from human nail material and its application to forensic casework. J Forensic Sci 1999;44:1053-6.
[Google Scholar]
|
| 49. |
Nakashima M, Tsuda M, Kinoshita A, Kishino T, Kondo S, Shimokawa O, et al. Precision of high-throughput single-nucleotide polymorphism genotyping with fingernail DNA: Comparison with blood DNA. Clin Chem 2008;54:1746-8.
[Google Scholar]
|
| 50. |
Nishiyori A, Fukuda K, Sata M, Tanikawa K. HBV DNA can be detected from nail clippings of HBs ag positive patients. Kurume Med J 2000;47:95-6.
[Google Scholar]
|
| 51. |
Dekio S, Jidoi J. Comparison of human hair and nail low-sulfur protein compositions on two-dimensional electrophoresis. J Dermatol 1989;16:284-8.
[Google Scholar]
|
| 52. |
Oimomi M, Hatanaka H, Ishikawa K, Kubota S, Yoshimura Y, Baba S, et al. Increased fructose-lysine of nail protein in diabetic patients. Klin Wochenschr 1984;62:477-8.
[Google Scholar]
|
| 53. |
Yoshida-Yamamoto S, Nishimura S, Okuno T, Rakuman M, Takii Y. Efficient DNA extraction from nail clippings using the protease solution from Cucumis melo. Mol Biotechnol 2010;46:41-8.
[Google Scholar]
|
| 54. |
Swisher EM, Wollan M, Mahtani SM, Willner JB, Garcia R, Goff BA, et al. Tumor-specific p53 sequences in blood and peritoneal fluid of women with epithelial ovarian cancer. Am J Obstet Gynecol 2005;193:662-7.
[Google Scholar]
|
| 55. |
Taback B, Hoon DS. Circulating nucleic acids and proteomics of plasma/serum: Clinical utility. Ann N Y Acad Sci 2004;1022:1-8.
[Google Scholar]
|
| 56. |
Salani R, Davidson B, Fiegl M, Marth C, Müller-Holzner E, Gastl G, et al. Measurement of cyclin E genomic copy number and strand length in cell-free DNA distinguish malignant versus benign effusions. Clin Cancer Res 2007;13:5805-9.
[Google Scholar]
|
| 57. |
Herrera LJ, Raja S, Gooding WE, El-Hefnawy T, Kelly L, Luketich JD, et al. Quantitative analysis of circulating plasma DNA as a tumor marker in thoracic malignancies. Clin Chem 2005;51:113-8.
[Google Scholar]
|
| 58. |
Inoue T, Kizawa K, Ito M. Characterization of soluble protein extracts from keratinized tissues: Identification of ubiquitin universally distributed in hair, nail, and stratum corneum. Biosci Biotechnol Biochem 2001;65:895-900.
[Google Scholar]
|
| 59. |
Giasuddin AS, Jhuma KA, Haq AM. Applications of free circulating nucleic acids in clinical medicine: Recent advances. Bangladesh Med Res Counc Bull 2008;34:26-32.
[Google Scholar]
|
| 60. |
Tong YK, Lo YM. Diagnostic developments involving cell-free (circulating) nucleic acids. Clin Chim Acta 2006;363:187-96.
[Google Scholar]
|
| 61. |
Pinzani P, Salvianti F, Pazzagli M, Orlando C. Circulating nucleic acids in cancer and pregnancy. Methods 2010;50:302-7.
[Google Scholar]
|
| 62. |
Zachariah R, Schmid S, Radpour R, Buerki N, Fan AX, Hahn S, et al. Circulating cell-free DNA as a potential biomarker for minimal and mild endometriosis. Reprod Biomed Online 2009;18:407-11.
[Google Scholar]
|
| 63. |
Zitt M, Müller HM, Rochel M, Schwendinger V, Zitt M, Goebel G, et al. Circulating cell-free DNA in plasma of locally advanced rectal cancer patients undergoing preoperative chemoradiation: A potential diagnostic tool for therapy monitoring. Dis Markers 2008;25:159-65.
[Google Scholar]
|
| 64. |
Dawber RP. The ultrastructure and growth of human nails. Arch Dermatol Res 1980;269:197-204.
[Google Scholar]
|
| 65. |
Fleckman P. Anatomy and physiology of the nail. Dermatol Clin 1985;3:373-81.
[Google Scholar]
|
| 66. |
Orentreich N, Markofsky J, Vogelman JH. The effect of aging on the rate of linear nail growth. J Invest Dermatol 1979;73:126-30.
[Google Scholar]
|
| 67. |
Samanidou V, Pantazidou K, Kovatsi L, Njau S, Livanos A. A simple HPLC method for the simultaneous determination of two selective serotonin reuptake inhibitors and two serotonin-norepinephrine reuptake inhibitors in hair, nail clippings, and cerebrospinal fluid. J Sep Sci 2012;35:839-45.
[Google Scholar]
|
| 68. |
Montgomery GW, Campbell MJ, Dickson P, Herbert S, Siemering K, Ewen-White KR, et al. Estimation of the rate of SNP genotyping errors from DNA extracted from different tissues. Twin Res Hum Genet 2005;8:346-52.
[Google Scholar]
|
| 69. |
Agalliu I, Schweitzer PA, Leanza SM, Burk RD, Rohan TE. Illumina DNA test panel-based genotyping of whole genome amplified-DNA extracted from hair samples: Performance and agreement with genotyping results from genomic DNA from buccal cells. Clin Chem Lab Med 2009;47:516-22.
[Google Scholar]
|
| 70. |
Verbeek NE, van Kempen M, Gunning WB, Renier WO, Westland B, Lindhout D, et al. Adults with a history of possible dravet syndrome: An illustration of the importance of analysis of the SCN1A gene. Epilepsia 2011;52:e23-5.
[Google Scholar]
|
| 71. |
Miller CJ, Cheung M, Sharma A, Clarke L, Helm K, Mauger D, et al. Method of mutation analysis may contribute to discrepancies in reports of (V599E) BRAF mutation frequencies in melanocytic neoplasms. J Invest Dermatol 2004;123:990-2.
[Google Scholar]
|
| 72. |
Lane JA, Noble JA. Maximizing deoxyribonucleic acid yield from dried blood spots. J Diabetes Sci Technol 2010;4:250-4.
[Google Scholar]
|
| 73. |
Mulot C, Stücker I, Clavel J, Beaune P, Loriot MA. Collection of human genomic DNA from buccal cells for genetics studies: Comparison between cytobrush, mouthwash, and treated card. J Biomed Biotechnol 2005;2005:291-6.
[Google Scholar]
|
| 74. |
Barker PE, Murthy M. Biomarker validation for aging: Lessons from mtDNA heteroplasmy analyses in early cancer detection. Biomark Insights 2009;4:165-79.
[Google Scholar]
|
| 75. |
Tsuboi R, Okeke CN, Inoue A, Yamazaki M, Hiruma M, Ogawa H, et al. Identification and viability assessment of dermatophytes infecting nail based on quantitative PCR of dermatophyte actin (ACT) mRNA. Nihon Ishinkin Gakkai Zasshi 2002;43:91-3.
[Google Scholar]
|
| 76. |
Baudelet M, Yu J, Bossu M, Jovelet J, Wolf JP, Amodeo T. Discrimination of microbiological samples using femtosecond laser-induced breakdown spectroscopy. Appl Phys Lett 2006;89:16903-6.
[Google Scholar]
|
| 77. |
Rehse SJ, Jeyasingham N, Diedrich J, Palchaudhuri S. A membrane basis for bacterial identification and discrimination using laser-induced breakdown spectroscopy. J Appl Phys 2009;105:102034-13.
[Google Scholar]
|
| 78. |
Samek, Liska M, Kaiser J, Beddows DC, Telle HH, Kukhlevsky SV. Clinical application of laser-induced breakdown spectroscopy to the analysis of teeth and dental materials. J Clin Laser Med Surg 2000;18:281-9.
[Google Scholar]
|
| 79. |
Corsi M, Cristoforetti G, Hidalgo M, Legnaioli S, Palleschi V, Salvetti A, et al. Application of laser-induced breakdown spectroscopy technique to hair tissue mineral analysis. Appl Opt 2003;42:6133-7.
[Google Scholar]
|
| 80. |
Kasem MA, Russo RE, Harith MA. Influence of biological degradation and environmental effects on the interpretation of archeological bone samples with laser-induced breakdown spectroscopy. J Anal At Spectrom 2011;26:1733-9.
[Google Scholar]
|
| 81. |
Hosseinimakarem Z, Tavassoli SH. Analysis of human nails by laser-induced breakdown spectroscopy. J Biomed Opt 2011;16:057002.
[Google Scholar]
|
| 82. |
Bahreini M, Hosseinimakarem Z, Tavassoli HS. A study of association between fingernail elements and osteoporosis by laser-induced breakdown spectroscopy. J Appl Phys 2012;112: 054701.
[Google Scholar]
|
| 83. |
Shi Y, Zhang J, Lu L, Shao B. Determination of triclosan and triclocarban in human nails by solid-phase extraction and ultra performance liquid chromatography-tandem mass spectrometry. Se Pu 2013;31:1040-5.
[Google Scholar]
|
| 84. |
Pearce DC, Dowling K, Gerson AR, Sim MR, Sutton SR, Newville M, et al. Arsenic microdistribution and speciation in toenail clippings of children living in a historic gold mining area. Sci Total Environ 2010;408:2590-9.
[Google Scholar]
|
| 85. |
Gherase MR, Desouza ED, Farquharson MJ, McNeill FE, Kim CY, Fleming DE. X-ray fluorescence measurements of arsenic micro-distribution in human nail clippings using synchrotron radiation. Physiol Measu 2013;34:1163.
[Google Scholar]
|
| 86. |
Hopps HC. The biologic bases for using hair and nail for analyses of trace elements. Sci Total Environ 1977;7:71-89.
[Google Scholar]
|
| 87. |
Baran R, Dawber RP, Haneke E, Tosti A, Bristow I, editors. A Text Atlas of Nail Disorders: Techniques in Investigation and Diagnosis. 3rd ed. London: Martin Dunitz, Taylor & Francis Group; 2005.
[Google Scholar]
|
| 88. |
Sukumar A. Human nails as a biomarker of element exposure. Rev Environ Contam Toxicol 2006;185:141-77.
[Google Scholar]
|
| 89. |
Kaminska-Winciorek G, Deja G, Polańska J, Jarosz-Chobot P. Diabetic microangiopathy in capillaroscopic examination of juveniles with diabetes type 1. Postepy Hig Med Dosw (Online) 2012;66:51-9.
[Google Scholar]
|
| 90. |
Kishabongo AS, Katchunga P, Van Aken EH, Speeckaert MM, Lagniau S, Husein D, et al. Glycated nail proteins: A new approach for detecting diabetes in developing countries. Trop Med Int Health 2014;19:58-64.
[Google Scholar]
|
| 91. |
O'Rorke MA, Cantwell MM, Abnet CC, Brockman AJ, Murray LJ; FINBAR Study Group. Toenail trace element status and risk of Barrett's oesophagus and oesophageal adenocarcinoma: Results from the FINBAR study. J Cancer 2012;131:1882-91.
[Google Scholar]
|
| 92. |
Jabłecka A, Olszewski J, Marzec E. Dielectric properties of keratin-water system in diabetic and healthy human fingernails. J Non Cryst Solids 2009;355:2456-60.
[Google Scholar]
|
| 93. |
Nasli-Esfahani E, Faridbod F, Larijani B, Ganjali MR, Norouzi P. Trace element analysis of hair, nail, serum and urine of diabetes mellitus patients by inductively coupled plasma atomic emission spectroscopy. Iran J Diabetes Lipid Disord 2011;10:1-9.
[Google Scholar]
|
| 94. |
Mandal BK, Ogra Y, Anzai K, Suzuki KT. Speciation of arsenic in biological samples. Toxicol Appl Pharmacol 2004;198:307-18.
[Google Scholar]
|
| 95. |
Karagas MR, Morris JS, Weiss JE, Spate V, Baskett C, Greenberg ER, et al. Toenail samples as an indicator of drinking water arsenic exposure. Cancer Epidemiol Biomarkers Prev 1996;5:849-52.
[Google Scholar]
|
| 96. |
Button M, Jenkin GR, Harrington CF, Watts MJ. Human toenails as a biomarker of exposure to elevated environmental arsenic. J Environ Monit 2009;11:610-7.
[Google Scholar]
|
| 97. |
Lu G, Xu H, Chang D, Wu Z, Yao X, Zhang S, et al. Arsenic exposure is associated with DNA hypermethylation of the tumor suppressor gene p16. J Occup Med Toxicol 2014;9:42.
[Google Scholar]
|
| 98. |
Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett 2002;133:1-16.
[Google Scholar]
|
| 99. |
Shi H, Hudson LG, Liu KJ. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radic Biol Med 2004;37:582-93.
[Google Scholar]
|
| 100. |
Wilhelm M, Pesch B, Wittsiepe J, Jakubis P, Miskovic P, Keegan T, et al. Comparison of arsenic levels in fingernails with urinary as species as biomarkers of arsenic exposure in residents living close to a coal-burning power plant in Prievidza district, Slovakia. J Expo Anal Environ Epidemiol 2005;15:89-98.
[Google Scholar]
|
| 101. |
MacDonell HL, Bialousz LF. Evaluation of human fingernails as a means of personal identification. In: Wecht C, editor. Legal Medicine Annual. New York: Appleton-Century-Crofts; 1972. p. 135-43.
[Google Scholar]
|
| 102. |
Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat 2009;214:516-59.
[Google Scholar]
|
| 103. |
Matte M, Williams L, Frappier R, Newman J. Prevalence and persistence of foreign DNA beneath fingernails. Forensic Sci Int Genet 2012;6:236-43.
[Google Scholar]
|
| 104. |
Brown HG, Perrett D. Detection of doping in sport: Detecting anabolic-androgenic steroids in human fingernail clippings. Med Leg J 2011;79:67-9.
[Google Scholar]
|
| 105. |
Kim JY, Cheong JC, Kim MK, Lee JI, In MK. Simultaneous determination of amphetamine-type stimulants and cannabinoids in fingernails by gas chromatography-mass spectrometry. Arch Pharm Res 2008;31:805.
[Google Scholar]
|
| 106. |
Kim JY, Shin SH, In MK. Determination of amphetamine-type stimulants, ketamine and metabolites in fingernails by gas chromatography-mass spectrometry. Forensic Sci Int 2010;194:108-14.
[Google Scholar]
|
| 107. |
Lin DL, Yin RM, Liu HC, Wang CY, Liu RH. Deposition characteristics of methamphetamine and amphetamine in fingernail clippings and hair sections. J Anal Toxicol 2004;28:411-7.
[Google Scholar]
|
| 108. |
Suzuki O, Hattori H, Asano M. Nails as useful materials for detection of methamphetamine or amphetamine abuse. Forensic Sci Int 1984;24:9.
[Google Scholar]
|
| 109. |
Lemos NP, Anderson RA, Robertson JR. The analysis of methadone in nail clippings from patients in a methadone-maintenance program. J Anal Toxicol 2000;24:656.
[Google Scholar]
|
| 110. |
Kim JY, Cheong JC, Lee JI, Son JH, In MK. Rapid and simple GC-MS method for determination of psychotropic phenylalkylamine derivatives in nails using micro-pulverized extraction. J Forensic Sci 2012;57:228.
[Google Scholar]
|
| 111. |
Garside D, Ropero-Miller JD, Goldberger BA, Hamilton WF, Maples WR. Identification of cocaine analytes in fingernail and toenail specimens. J Forensic Sci 1998;43:974-9.
[Google Scholar]
|
| 112. |
Lemos NP, Anderson RA, Robertson JR. Nail analysis for drugs of abuse: Extraction and determination of cannabis in fingernails by RIA and GC-MS. J Anal Toxicol 1999;23:147-52.
[Google Scholar]
|
| 113. |
Morini L, Colucci M, Ruberto M, Groppi A. Determination of ethyl glucuronide in nails by liquid chromatography tandem mass spectrometry as a potential new biomarker for chronic alcohol abuse and binge drinking behavior. Anal Bioanal Chem 2012;402:1865.
[Google Scholar]
|
| 114. |
Jones J, Jones M, Plate C, Lewis D, Fendrich M, Berger L, et al. Liquid chromatography-tandem mass spectrometry assay to detect ethyl glucuronide in human fingernail: Comparison to hair and gender differences. Am J Anal Chem 2012;3:83.
[Google Scholar]
|
| 115. |
Grover C, Chaturvedi UK, Reddy BS. Role of nail biopsy as a diagnostic tool. Indian J Dermatol Venereol Leprol 2012;78:290-8.
[Google Scholar]
|
| 116. |
Tirado-González M, González-Serva A. The nail plate biopsy may pick up gout crystals and other crystals. Am J Dermatopathol 2011;33:351-3.
[Google Scholar]
|
| 117. |
Al-Delaimy WK, Stampfer MJ, Manson JE, Willett WC. Toenail nicotine levels as predictors of coronary heart disease among women. Am J Epidemiol 2008;167:1342-8.
[Google Scholar]
|
| 118. |
Kumar A, Garg S, Hanmandlu M. Biometric authentication using finger nail plates. Expert systems with applications 2014;41:373-86.
[Google Scholar]
|
| 119. |
Garg S, Kumar A, Hanmandlu M. Finger nail plate: A new biometric identifier. Int J Compt Inf Syst Ind Manage Appl 2014;6:126-38.
[Google Scholar]
|
| 120. |
Garg S, Kumar A, Hanmandlu M. Biometric Authentication Using Finger Nail Surface. In Intelligent Systems Design and Applications (ISDA), 2012 12th International Conference on 2012 November 27, IEEE; 2012. p. 497-502.
[Google Scholar]
|
| 121. |
Topping A, Kuperschmidt V, Gormley A; Inventors; Topping, Allen, Kuperschmidt, Vladimir, Gormley, Austin, Assignee. Method and Apparatus for the Automated Identification of Individuals by the Nail Beds of their Fingernails. United States Patent US 5,751,835; May 1998.
[Google Scholar]
|
| 122. |
Runne U, Orfanos CE. The human nail: Structure, growth and pathological changes. Curr Probl Dermatol 1981;9:102-49.
[Google Scholar]
|
| 123. |
Baden HP. The physical properties of nail. J Invest Dermatol 1970;55:115-22.
[Google Scholar]
|
| 124. |
Diaz AA, Boehm AF, Rowe WF. Comparison of fingernail ridge patterns of monozygotic twins. J Forensic Sci 1990;35:97-102.
[Google Scholar]
|
| 125. |
Robson JR. Hardness of finger nails in well-nourished and malnourished populations. Br J Nutr 1974;32:389-94.
[Google Scholar]
|
| 126. |
Reyes RA, Romanyukha A, Olsen C, Trompier F, Benevides LA. Electron paramagnetic resonance in irradiated fingernails: Variability of dose dependence and possibilities of initial dose assessment. Radiat Environ Biophys 2009;48:295-310.
[Google Scholar]
|
| 127. |
Reyes RA, Trompier F, Romanyukha A. Study of the stability of EPR signals after irradiation of fingernail samples. Health Phys 2012;103:175-80.
[Google Scholar]
|
| 128. |
He X, Swarts SG, Demidenko E, Flood AB, Grinberg O, Gui J, et al. Development and validation of an ex vivo electron paramagnetic resonance fingernail biodosimetric method. Radiat Prot Dosimetry 2014;159:172-81.
[Google Scholar]
|
| 129. |
Swartz HM, Flood AB, Gougelet RM, Rea ME, Nicolalde RJ, Williams BB, et al. Acritical assessment of biodosimetry methods for large-scale incidents. Health Phys 2010;98:95-108.
[Google Scholar]
|
| 130. |
González AJ. Lauriston S. Taylor Lecture: Radiation protection in the aftermath of a terrorist attack involving exposure to ionizing radiation. Health Phys 2005;89:418-46.
[Google Scholar]
|
Fulltext Views
10,331
PDF downloads
2,973





