Translate this page into:
Therapeutic plasma exchange as a crisis option in severe pemphigus vulgaris
2 Department of Nephrology, JIPMER, Puduchery - 605006, India
Correspondence Address:
Rashmi Kumari
Assistant Professor, Department of Dermatology and STD, JIPMER, Puduchery - 605006
India
| How to cite this article: Ranugha P, Kumari R, Kartha LB, Parameswaran S, Thappa DM. Therapeutic plasma exchange as a crisis option in severe pemphigus vulgaris. Indian J Dermatol Venereol Leprol 2012;78:508-510 |
Sir,
Although conventional therapy with steroids and immunosuppressive agents is able to control the disease process in many patients with pemphigus vulgaris (PV), in some, these are ineffective or are contraindicated, wherein the role of various immunomodulatory drugs and procedures comes into play. Intravenous immunoglobulins (IVIg), rituximab, therapeutic plasma exchange (TPE), immunoadsorption and extracorporeal photopheresis can achieve rapid control of disease activity but need combination with steroids and other immunosuppressives or maintenance sessions for sustained remission. Considering the high cost of these procedures and the risk of infection-related complications, their use is limited to severe pemphigus wherein rapid control of disease is desired or in recalcitrant cases not responding to conventional therapy. We herewith report a case of severe PV, who was showing slower response to dexamethasone azathioprine pulse therapy and was successfully treated with TPE, an option hardly being considered in the Indian scenario.
A 24-year-old married female came to the hospital with blisters and erosions all over the body and in the oral mucosa of one-month duration. On physical examination, she was febrile with numerous flaccid blisters and erosions involving the oral mucosa and more than 60% of the body surface area, some covered with slough. Perilesional Nikolsky sign was positive. Tzanck smear, skin biopsy and direct immunofluorescence confirmed the diagnosis of PV. She was put on oral prednisolone at a dose of 1 mg/ kg/day along with oral azathioprine at a dose of 50 mg/day on admission for 7 days. Cyclophosphamide was not used as a steroid sparing agent considering her reproductive age and as she was nulliparous. Subsequently, she was given intravenous dexamethasone pulse (120 mg of dexamethasone) on three consecutive days, once routine blood investigations were found to be normal and blood and urine cultures were sterile, thereby ruling out systemic sepsis. Intravenous antibiotics were given according to the pus culture and sensitivity reports from the cutaneous erosions. She was maintained on 1 mg/kg of oral prednisolone with 50 mg of azathioprine daily after the pulse. One week after the pulse, she did not show any improvement and developed fever spikes of 102°F every day. Blood culture grew Enterobacter and Enterococcus faecalis, while the pus culture from erosions grew Staphylococcus aureus, Proteus mirabilis and Klebsiella pneumoniae. Based on sensitivity reports, she was started on intravenous vancomycin and meropenem. As the erosions were persisting and the disease was still active, the prednisolone dose was hiked up to 1.5 mg/kg/day three days after start of antibiotics. Two weeks after the dexamethasone azathioprine pulse, the erosions still persisted and did not show any evidence of reepithelialization, Nikolsky sign was still positive [Figure - 1], and she continued to develop few new blisters. Her albumin levels fell to 2.1 mg% and she developed edema of hands and feet. She continued to develop fever spikes of 100°F. As she was in sepsis with persistent high grade fever, we could not consider giving her ′Interval pulse′ of intravenous dexamethasone, IVIgs or rituximab. Considering all the above factors, she was given six sessions of TPE over a period of 2 weeks with a gap of 2 to 3 days between the sessions. Two liters of plasma was removed using a plasma filter attached to a standard dialysis machine and replaced with 1 liter of normal saline and 1 liter of fresh frozen plasma. The procedure was done by a nephrologist in the nephrology department of our hospital. As plasmapheresis is being done routinely in our hospital for patients of myasthenia gravis and Guillain Barre syndrome, the procedure was done free of cost. After the second session of TPE, Nikolsky′s sign became negative and no new blisters appeared. The erosions showed 50% reepithelialization after three sessions [Figure - 2] and around 95% healing after the 5th cycle of TPE [Figure - 3]. The oral erosions also healed completely after the 5 th cycle of TPE, though a few erosions on the posterior aspect of both the thighs alone persisted. A week later her skin lesions healed completely. Serum antibody levels before and after plasmapheresis sessions could not be done as the patient could not afford it. She was maintained on monthly intravenous dexamethasone pulse along with daily oral azathioprine (50 mg once daily). The interpulse oral prednisolone was gradually tapered and stopped over a period of 4 months. She continued to be in remission and at present has completed 7 monthly dexamethasone azathioprine pulses.
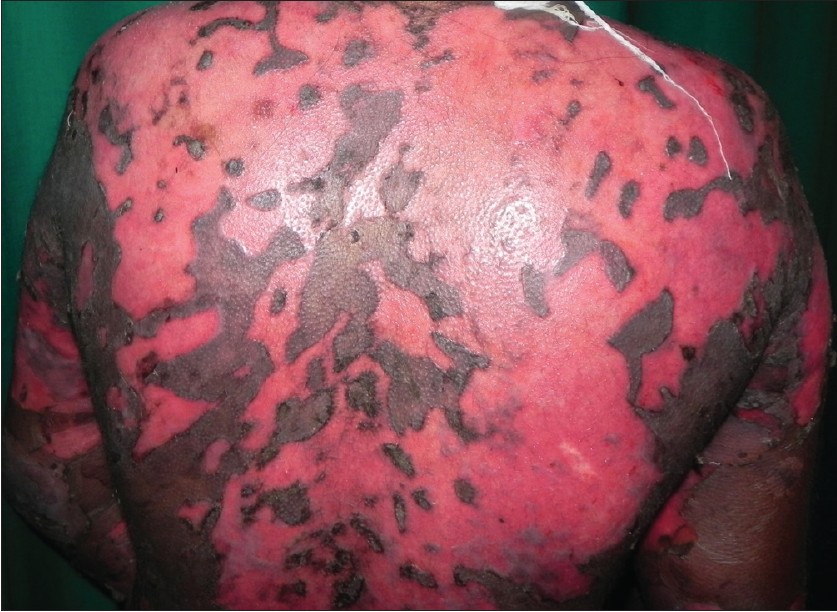 |
| Figure 1: Extensive non-healing erosions over the back two weeks after the dexamethasone azathioprine pulse |
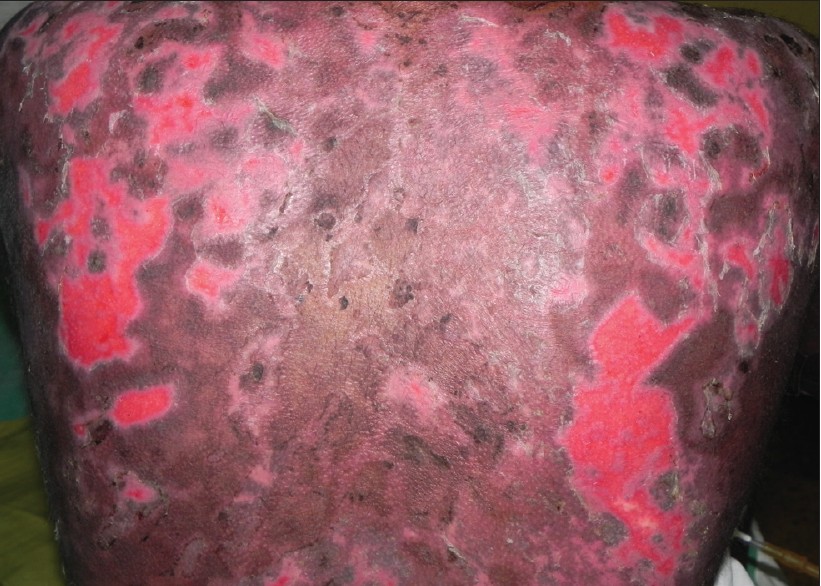 |
| Figure 2: Erosions over the back showing reepithelialization after three sessions of TPE |
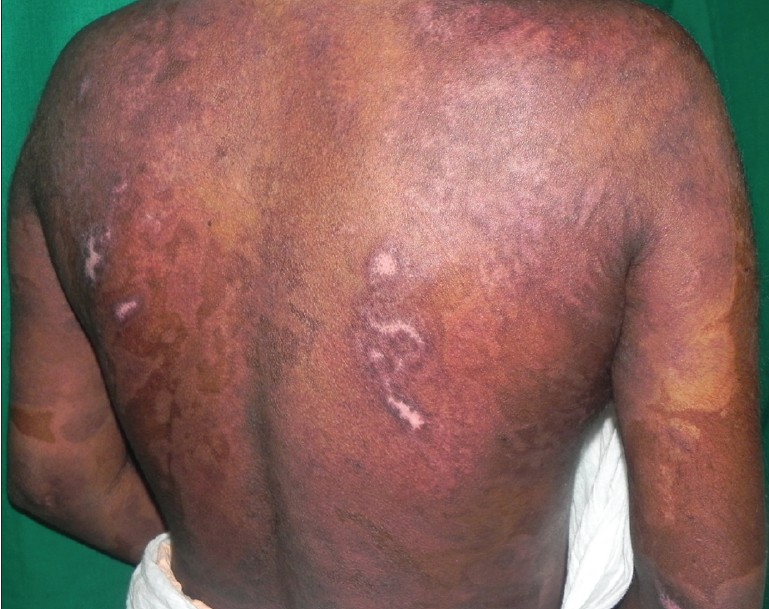 |
| Figure 3: Complete healing of erosions over the back after five sessions of TPE |
TPE/plasmapheresis is an extracorporeal blood purification technique in which the blood is continuously removed from the patient and separated into cellular components and plasma; the cellular compartments are returned to the patients along with replacement fluid-like albumin. It is used for various autoimmune diseases like Goodpasture syndrome, myasthenia gravis, Guillain-Barre syndrome, Refsum′s disease, familial hypercholesterolemia, systemic lupus erythematosus, PV and bullous pemphigoid. The first therapeutic implications of TPE in PV were described by Cotterill in 1978. [1] Since then, plasma exchange has been described as an effective adjuvant therapy in PV patients in controlling disease activity by reducing serum levels of autoantibodies. [2] Plasma exchange can be performed using centrifugation device used in blood banks or by the more effective membrane plasma separation (MPS). MPS utilizes a highly permeable membrane and a standard dialysis machine used in its ultrafiltration mode, dialysis-bypass mode. Even though, there is no standardized protocol for the number and frequency of sessions, it is commonly believed that four or five plasma exchanges, each exchange consisting of 1 to 1.5 plasma volumes over a period of 7 to 10 days constitutes an adequate short-term therapy, [3] to remove 90 percent of the total initial body immunoglobulin burden. In disorders for which TPE is an effective therapy, the number of sessions required is usually decided based on the clinical response as in our case. Albumin, fresh frozen plasma (FFP) or a combination of albumin and saline are the replacement fluids used. We used FFP in our patient without any adverse effects. The late rebound increase in serum antibody levels which is observed 1 to 2 weeks after [4] was suppressed by giving intravenous dexamethasone azathioprine pulse after the last session in our patient.
TPE is relatively safe and the risk of infection associated with it is mainly due to the steroids and immunosuppressives given along with it. Other transient and minor adverse effects of plasma exchange that have been reported include thrombocytopenia, hypogammaglobulinemia, fluid overload leading to hypertension and pulmonary edema, hypoproteinemia, anemia, leukopenia and hypocalcemia. [4] Due to the rapid fluid shift occurring as a result of removal of proteins, which maintain the osmotic pressure, it can lead to severe problems in patients with compromised cardiac function. Outcome of therapy in our case was similar to that reported by Sagi et al., [5] who studied the role of TPE as steroid sparing therapy in 7 patients and found 86% response rate with three cycles of TPE per week along with monthly IV dexamethasone pulse, without any major adverse effects. We could safely practice TPE in our patient despite evidence of sepsis. A similar observation was made by Saraceno et al. [6] in a 63-year-old male who developed Nocardia asteroides infection when on conventional therapy and responded well to TPE. While immunoadsorption is far superior to plasmapheresis in terms of efficacy and safety, the high cost of the adsorbers is the major limiting factor. TPE, on the other hand, can be done in any center having facilities for dialysis. The cost of the procedure is mainly due to the cost of the plasma filter and the albumin, which is used for replacement. Even though the cost per session in the Indian setting can be anywhere between 10,000 and 15,000, this can definitely be reduced by using FFP instead of albumin as in our case and by continuous heparinization, thereby one plasma filter can be used for a maximum of three sessions, further minimizing the cost. While the cost of four injections of rituximab or one cycle of IVIg could be approximately 1.2 to 1.5 lakhs in India, five to six sessions of plasmapheresis will cost less than half of the same.
| 1. |
Cotterill JA, Barker DJ, Millard LG. Plasma exchange in the treatment of pemphigus vulgaris. Br J Dermatol 1978;98:243.
[Google Scholar]
|
| 2. |
Tan-Lim R, Bystryn JC. Effect of plasmapheresis therapy on circulating levels of pemphigus antibodies. J Am Acad Dermatol 1990;22:35-40.
[Google Scholar]
|
| 3. |
Shumak KH, Rock GA. Therapeutic plasma exchange. N Engl J Med 1984;310:762-71.
[Google Scholar]
|
| 4. |
Yeh SW, Sami N, Ahmed RA. Treatment of pemphigus vulgaris: Current and emerging options. Am J Clin Dermatol 2005;6:327- 42.
[Google Scholar]
|
| 5. |
Sagi L, Baum S, Gendelman V, Trau H, Barzilai A. The role of therapeutic plasma exchange in pemphigus vulgaris. J Eur Acad Dermatol Venereol 2011;25:82-6.
[Google Scholar]
|
| 6. |
Saraceno R, Ruzzetti M, Lanti A, Marinacci M, Chimenti S. Therapeutic options in an immunocompromised patient with pemphigus vulgaris: Potential interest of plasmapheresis and extracorporeal photochemotherapy. Eur J Dermatol 2008;18:354-6.
[Google Scholar]
|
Fulltext Views
5,755
PDF downloads
2,042





