Translate this page into:
Therapeutic success of tofacitinib in granuloma annulare: A retrospective case series of 15 patients
Corresponding author: Dr. Vinay Keshavamurthy, Department of Dermatology, PGIMER, Chandigarh, India. vinay.keshavmurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dev A, Keshavamurthy V, Chatterjee D. Therapeutic success of tofacitinib in granuloma annulare: A retrospective case series of 15 patients. Indian J Dermatol Venereol Leprol. 2025;91:65-70. doi: 10.25259/IJDVL_215_2024
Abstract
Background
Granuloma annulare (GA) is a necrobiotic granulomatous disorder that may sometimes be resistant to treatment, especially the generalised form. Tofacitinib has recently shown promise in the treatment of non-infective granulomatous dermatosis.
Objectives
In this study, we aimed to evaluate the response of generalised GA to oral tofacitinib.
Methods
This was a retrospective case series in patients of generalised GA who were treated with oral tofacitinib 5 mg twice a day in a tertiary care centre in north India. Baseline clinical details and histopathological findings were reviewed. Treatment response was noted in the form of clearance of lesions (complete or partial) along with the time taken to achieve the maximum response.
Results
A total of 15 patients of generalised GA were included in this study, amongst whom nine patients were resistant to conventional therapies whilst the remaining were treatment naïve. Complete clearance of lesions was noted in 11 patients at a mean treatment duration of 4.4 ± 2.1 months whereas clearance was partial in four, with a mean follow-up duration post- treatment in patients who had partial clearance, which is 7.3 ± 2.8 month, with a reduction in erythema and infiltration in those lesions. Adverse effects in the form of hyperlipidemia were observed in two patients.
Conclusion
Tofacitinib, a JAK-STAT inhibitor is beneficial in treating GA, especially in those with generalised and recalcitrant disease.
Keywords
granuloma annulare
tofacitinib
JAK-STAT
Introduction
Granuloma annulare (GA) is a necrobiotic granulomatous disorder, which presents as annular erythematous plaques.1 Even though few studies have suggested that GA is self-remitting, treatment is generally required especially in patients with generalised disease. Various treatment modalities ranging from topical steroids to TNF-alpha inhibitors have been used with variable success.2,3 JAK-STAT inhibitors have recently shown promise in the treatment of various granulomatous conditions. In this study, we report our experience of treating 15 patients of generalised GA with oral tofacitinib.
Patients and methods
This was a retrospective case series of patients with biopsy proven generalised GA treated and followed-up at a tertiary care centre. Patients of generalised GA seen during August 2022 to December 2023 who had received oral tofacitinib for at least 3 months were included in this case series [Table 1]. Baseline details including history, examination, and histopathological evaluation were recorded and reviewed. Haematological investigations including complete blood count, liver and renal function tests, thyroid function test, fasting lipid profile, hepatitis B surface antigen, hepatitis C, HIV, chest X-ray, Mantoux test and urine routine microscopy were performed at baseline and recorded. Complete blood count, liver function test, and fasting lipid profile were repeated every month during the treatment period. Treatment response was noted in the form of clearance of lesions (complete or partial) along with the time taken to achieve the maximum response. Any adverse effects that developed during the treatment period were noted. The data was recorded and compiled using Microsoft Excel (Office 365, Washington, USA) and data analysis was performed using Statistical Package for Social Sciences (SPSS v29, IBM, New York, USA).
| Serial No. | Age in years/Sex | Comorbidities | Previous therapies | Morphology of GA | Diameter of largest lesion in centimeters | Dose of tofacitinib | Treatment duration | Treatment response | Time taken for complete clearance in months | Duration of tapering | Duration of follow-up | Sequelae | Adverse effect of tofacitinib |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 68/F | DM, hypertension | None | Annular plaque | 15 | 5 mg twice a day | 12 months | Partial | - | Changed to apremilast after 12 months | 1.5 years | Atrophy | None |
| Patient 2 | 48/F | DM | Oral steroids, acitretin, doxycycline | Papules coalescing to form annular plaque | 8 | 5 mg twice a day | 4 months | Complete | 3 | 1 month | 6 months | PIH | None |
| Patient 3 | 56/F | None | None | Papules coalescing to form annular plaque | 2 | 5 mg twice a day | 4 months | Complete | 3 | 1 month | 4 months | None | None |
| Patient 4 | 57/F | DM, hypertension | Topical steroids | Papules coalescing to form annular plaque | 3 | 5 mg twice a day | 6 months | Complete | 5 | 1 month | 6 months | PIH | None |
| Patient 5 | 63/F | DM | None | Papules coalescing to form annular plaque | 5 | 5 mg twice a day | 8 months | Complete | 7 | 1 month | 9 months | PIH | None |
| Patient 6 | 60/F | DM, hypertension | HCQs, topical steroids, tacrolimus | Annular plaque | 5 | 5 mg twice a day | 6 months | Partial | - | - | 6 months | None | None |
| Patient 7 | 55/F | None | Isotretinoin, topical tacrolimus | Papular | 1 | 5 mg twice a day | 6 months | Complete | 5 | 1 month | 8 months | None | None |
| Patient 8 | 57/F | DM, hyperlipidemia | None | Papules coalescing to form annular plaque | 2.5 | 5 mg twice a day | 4 months | Complete | 3 | 1 month | 4 months | Atrophy | None |
| Patient 9 | 42/F | None | None | Papules coalescing to form annular plaque | 1.5 | 5 mg twice a day | 5 months | Complete | 4 | 1 month | 6 months | PIH | Hyperlipidemia |
| Patient 10 | 46/F | Hypertension, mild anemia, depression | None | Papules coalescing to form annular plaque | 2 | 5 mg twice a day | 3 months | Complete | 2 | 1 month | 5 months | None | None |
| Patient 11 | 63/F | Hypothyroidism | Topical steroids | Annular plaque | 4 | 5 mg twice a day | 5 months | Partial | - | - | 5 months | PIH | None |
| Patient 12 | 50/F | DM, mild anemia | HCQs, topical steroids, tacrolimus | Annular plaque | 4 | 5 mg twice a day | 6 months | Partial | - | Changed to methotrexate after 6 months | 7 months | Atrophy | None |
| Patient 13 | 34/M | None | Topical steroids | Papules coalescing to form annular plaque | 8 | 5 mg twice a day | 9 months | Complete | 8 | 1 month | 10 months | PIH | Hyperlipidemia |
| Patient 14 | 61/M | None | Topical steroids | Papular | 0.5 | 5 mg twice a day | 8 months | Complete | 7 | 1 month | 9 months | None | None |
| Patient 15 | 49/F | Hypothyroidism | Topical steroids | Papular | 1 | 5 mg twice a day | 3 months | Complete | 2 | 1 month | 12 months | PIH | None |
M- male; F-female; DM- Diabetes mellitus; HCQ- hydroxychloroquine; GA- granuloma annulare; PIH- post-inflammatory hyperpigmentation
Results
A total of 15 patients of generalised GA were included in this study, which included 13 females and 2 males [Table 1]. The mean age of this cohort was 53.9 ± 9.1 years, and the mean total duration of illness was 9.0 ± 8.6 months. Ten patients had one or more comorbidities, the most common being type 2 diabetes mellitus in seven, followed by hypertension in four, anaemia and hypothyroidism in two each, hyperlipidemia, and depressive disorder in one each. Nine patients had received prior treatment with modalities like topical steroids, topical tacrolimus, oral steroids, acitretin, isotretinoin, dapsone, doxycycline, and hydroxychloroquine, with minimal response, while the remaining six were treatment naïve. Various morphologies of GA were observed, the most common being erythematous papules coalescing to form annular non-scaly plaques in eight patients, followed by annular plaques in four and papules in three. The mean diameter of the largest lesion was 4.2 ± 3.8 cm with sizes of lesions ranging from 0.5 cm to 15 cm.
The diagnosis had been confirmed on histopathological examination in all patients which showed dermal necrobiotic epithelioid cell granulomas along with increased dermal mucin and varying amounts of inflammatory infiltrate. Baseline investigations were normal in all, except for mild anaemia in two patients.
Tofacitinib was administered at a dose of 5 mg twice a day. No other additional topical or systemic treatment other than emollients were allowed. The mean total duration of treatment was 5.9 ± 2.4 months. Complete clearance of lesions was observed in 11 patients at a mean duration of 4.4 ± 2.1 months (ranging from 2 months to 8 months) [Figures 1–4]. Clearance was partial in the remaining four, with a mean treatment duration of 7.3 ± 2.8 months, however, there was a significant reduction in erythema and infiltration in those lesions which did not completely resolve [Figure 5]. Post-treatment sequelae were observed in 10 patients, which included post inflammatory hyperpigmentation (n = 7) [Figure 1b] and dermal atrophy (n = 3) [Figure 5b]. Apart from hyperlipidemia (Patient 9 had total cholesterol levels of 245 mg/dl and LDL levels of 144 mg/dl after 1 month of tofacitinib; patient 13 had a total cholesterol level of 266 mg/dl, triglyceride levels of 335 mg/dl and LDL of 157 mg/dl after 3 months of tofacitinib) in two instances which was managed with lifestyle modifications, no other adverse effect to tofacitinib was observed in our study. In those who achieved complete clearance of lesions, tofacitinib was tapered to a dose of 5 mg once a day for a month and subsequently stopped. Amongst the patients who achieved partial clearance, two patients continue to receive tofacitinib and are currently under follow-up, while two patients were switched to apremilast and methotrexate, respectively [Table 1]. Two patients experienced recurrence of lesions 1 month and 3 months after stopping tofacitinib, which resolved promptly after re-administering tofacitinib for 1 month. Mean follow-up duration was 7.7 ± 3.7 months.
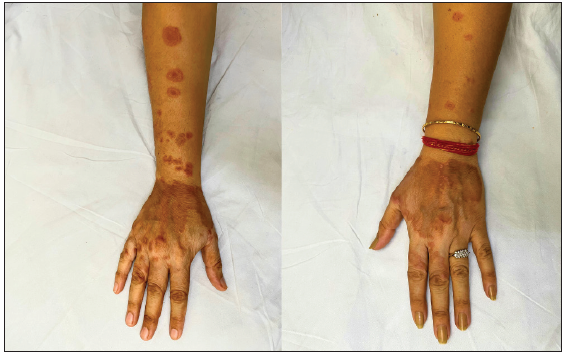
- Pre-treatment image of patient 2 showing annular erythematous plaques with central clearing present on bilateral forearms and dorsal of hands.
- Post-treatment image (at 3 months of therapy with tofacitinib) demonstrating complete clearance of lesions with post inflammatory hyperpigmentation.
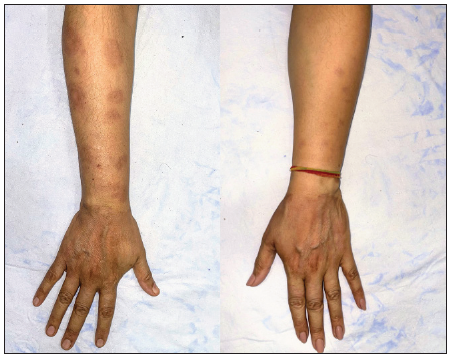
- Pre-treatment image of patient 4 showing annular erythematous to brownish papules and plaques with central clearing present on bilateral dorsa of hands.
- Post-treatment image (at 5 months) showing complete clearance of lesions.
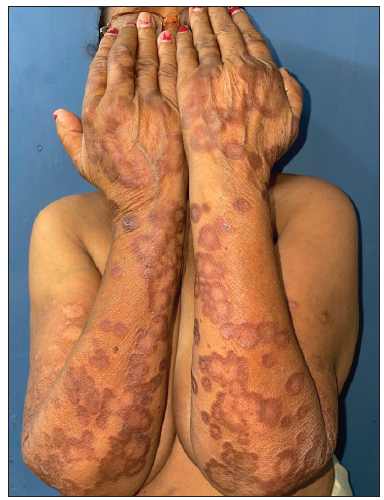
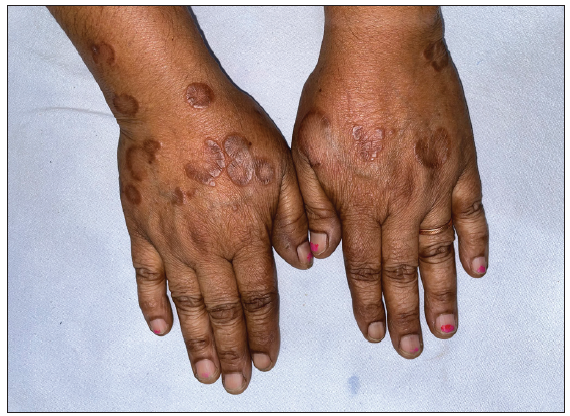
- Pre-treatment image of patient 5 showing annular erythematous to brownish plaques with central clearing present on bilateral forearms.
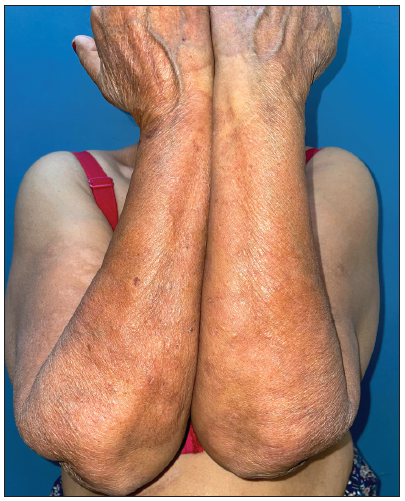
- Post-treatment image (at 7 months) showing complete resolution of lesions.
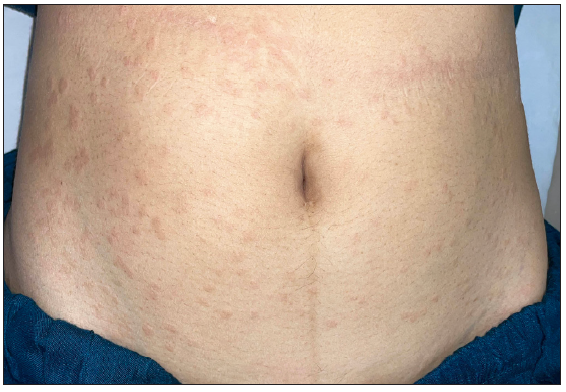
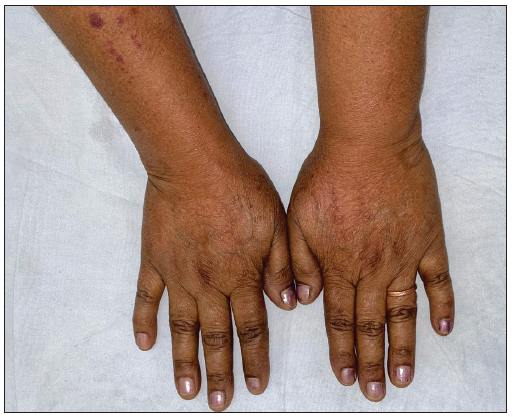
- Pre-treatment image of the patient 10 showing annular erythematous papules and plaques with central clearing on the abdomen.
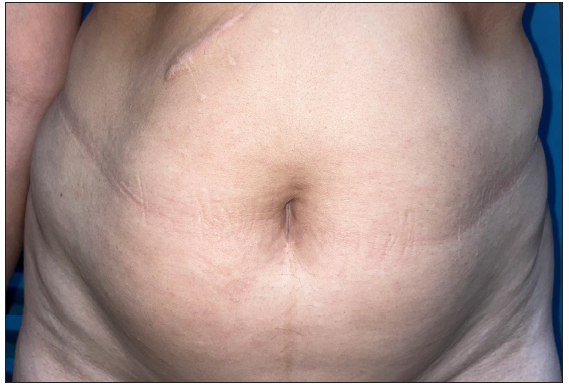
- Post-treatment image (at 2 months) showing complete clearance of lesions.
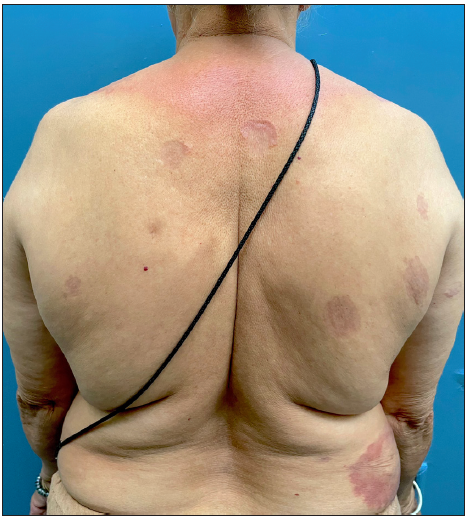
- Pre-treatment image of patient 1 showing annular erythematous plaques with central clearing and atrophy on the back.
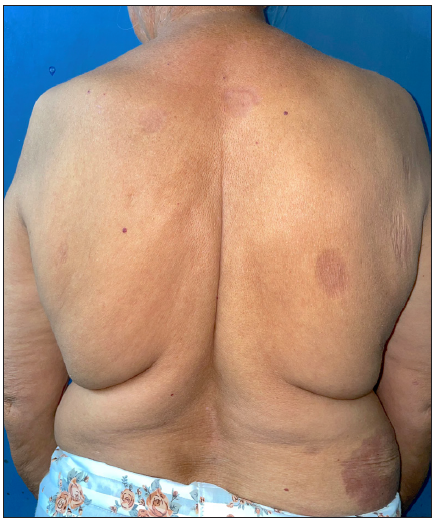
- Post-treatment image at 6 months of treatment with tofacitinib showing partial response.
Discussion
GA is a common necrobiotic dermatosis with a reported prevalence ranging from 0.04% to 0.1%. It predominantly affects middle-aged women, as observed in our study cohort.4,5 GA can be either localised or generalised; generalised GA refers to the presence of lesions on the trunk and either on the upper or lower extremities or both.6 Rare variants include perforating, patch, subcutaneous, and palmoplantar GA. Accurate diagnosis can sometimes be challenging due to similarities with other conditions like annular elastolytic giant cell granuloma and annular sarcoidosis.1 Necrobiotic granulomas which can either be palisading or interstitial along with dermal mucin are the hallmark histopathological feature of GA2 and was observed in all the patients in our series. Various conditions are associated with GA, including diabetes mellitus, hyperlipidemia, thyroid disorders, malignancies, HIV, and autoimmune diseases.2,3 In our cohort, 46.6% of patients had concomitant diabetes mellitus, which is higher than the reported average of 20% in GA patients.7
A multitude of treatment modalities have been tried in GA, ranging from the conventional agents like topical and oral corticosteroids, antimicrobials like tetracyclines and dapsone, antimalarials, isotretinoin, methotrexate, phototherapy to the newer agents like apremilast and biologics.3,8 The response rates to these agents vary across studies and often recalcitrant cases are encountered especially in patients with generalised GA.
The pathogenesis of GA involves T-cell dysregulation, with implications of Th1 and possibly Th2 pathways.3, 9,10 Recently, Wang et. al reported the activation of Th17 and the JAK-STAT pathway in GA.9 Elevated levels of cytokines like interferon-gamma (IFN-γ) and oncostatin M have been observed in GA patients, which decrease after treatment with tofacitinib, a JAK-STAT inhibitor. Cytokines such as IFN-γ, IL-21, and chemokines, released from T-cells activates macrophages and fibroblasts which leads to the formation of granulomas in GA. JAK-STAT inhibitors blocks this activation of macrophages and fibroblasts, thereby resulting in the resolution of granulomas.9
Damsky et al. demonstrated the activation of STAT 1 and 3 in cutaneous granulomas of sarcoidosis and GA, reporting subsequent improvement after treatment with tofacitinib.11 Wang et al. observed that three out of five patients achieved complete resolution of lesions and two had partial resolution at 6 months of treatment with tofacitinib.9 Other authors have observed similar response to tofacitinib in cases of recalcitrant generalised GA.12 Interestingly, 2% topical tofacitinib has also effectively cleared lesions of generalised GA within 3 months.13,14
Though topical and intralesional corticosteroids are considered as first-line therapy, they have limited efficacy in the treatment of generalised GA.3 Retrospective studies report modest responses to antimicrobials like doxycycline (28.6% response rate), whereas dapsone shows higher efficacy with a 54% response rate.3,15 Anti-malarials show a variable response, with response rates ranging from 35% to 79.6%.3 Methotrexate is an effective second-line therapy, with response rates of around 60%.16 Amongst the newer modalities, good improvement with apremilast has been noted in studies with response duration ranging from 2 months to 8 months of therapy.8 Amongst the biologics, TNF-alpha inhibitors show the most promise, with the complete clearance in patients receiving adalimumab and infliximab.17 In comparison to pre-existing therapies for GA, tofacitinib consistently demonstrates positive responses in all patients, with our study showing complete clearance in 73.3% of cases and partial clearance in others.
Although there are no specific guidelines regarding the optimal duration of tofacitinib treatment before considering non-response, most authors administer it for at least 6 months, with some patients have received treatment for over 9 months.18 Patients in our study who received tofacitinib for more than 6 months showed improvement within the initial 2–3 months, with complete lesion clearance at 7–8 months for specific cases (Patients 5, 13, 14). Based on our experience, patients can be administered tofacitinib for a minimum duration of 3 months before considering them non-responsive.
Though tofacitinib presents a promising avenue of therapy in GA, it is not devoid of potential adverse effects like increased risk of infections, raised liver enzymes, hyperlipidemia, hypothyroidism, bone marrow suppression, and adverse cardiovascular events.9,12 Given the higher age and association of GA patients with DM, thyroid disorders, and hyperlipidemia, they are more prone to develop the above-mentioned adverse effects. While hyperlipidemia was manageable in the two patients in our series, caution must be exercised in the form of prudent patient selection, baseline investigations, and stringent follow-up monitoring.
The limitation of this study is the retrospective design and lack of a post-treatment biopsy.
Conclusion
Tofacitinib, a JAK-STAT inhibitor is beneficial in the treatment of generalised GA. It can be considered as a promising treatment modality, especially in recalcitrant cases. Further controlled studies are required to elucidate the efficacy of tofacitinib as compared to conventional agents in the treatment of GA as well as to understand the role of the JAK-STAT pathway in granulomatous disorders.
Ethical approval
For this retrospective study, the data was retrieved from the hospital records and individual patient data was anonymised.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Granuloma annulare: Clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-65.
- [CrossRef] [PubMed] [Google Scholar]
- Granuloma annulare: Pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-79.
- [CrossRef] [PubMed] [Google Scholar]
- Granuloma annulare: An updated review of epidemiology, pathogenesis, and treatment options. Am J Clin Dermatol. 2022;23:37-50.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Incidence and prevalence of granuloma annulare in the United States. JAMA Dermatol. 2021;157:824.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of granuloma annulare in the United States: A cross‐sectional study in the all of us research program. Int J Dermatol. 2022;61
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Generalized granuloma annulare: Clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- [CrossRef] [PubMed] [Google Scholar]
- Association of granuloma annulare with type 2 diabetes, hyperlipidemia, autoimmune disorders, and hematologic malignant neoplasms. JAMA Dermatol. 2021;157:817.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Refractory generalized granuloma annulare treated with oral apremilast. JAMA Dermatol. 2019;155:1318.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-809.
- [CrossRef] [PubMed] [Google Scholar]
- Granuloma annulare skin profile shows activation of T-helper cell type 1, T-helper cell type 2, and Janus kinase pathways. J Am Acad Dermatol. 2020;83:63-70.
- [CrossRef] [PubMed] [Google Scholar]
- Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Treatment of granuloma annulare with tofacitinib. Australas J Dermatol. 2022;63:400-3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Treatment of granuloma annulare with tofacitinib 2% ointment. JAAD Case Rep. 2020;6:69-71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Generalized granuloma annulare: A widespread response to limited application of compounded 2% topical tofacitinib. JAAD Case Rep. 2020;6:1113-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Granuloma annulare: A retrospective series of 133 patients. Cutis. 2019;103:102-6.
- [PubMed] [Google Scholar]
- Methotrexate for generalized granuloma annulare: A 60% response rate in a retrospective case series of 15 patients. J Am Acad Dermatol. 2022;87:201-3.
- [CrossRef] [PubMed] [Google Scholar]
- The role of biologics in the treatment of chronic granuloma annulare. Int J Dermatol. 2019;58:622-6.
- [CrossRef] [PubMed] [Google Scholar]
- Improvement of granulomatous skin conditions with tofacitinib in three patients: A case report. SAGE Open Med Case Rep. 2021;9:2050313X2110394.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






