Translate this page into:
Thrombophilia in venous leg ulcers: A comparative study in early and later onset
2 Department of Immunohemotherapy, Hospital S�o Jo�o, EPE, Porto, Portugal
Correspondence Address:
Ana M Calistru
Department of Dermatology and Venereology, Hospital S�o Jo�o, EPE, Alameda Professor Hern�ni Monteiro, 4200 319, Porto
Portugal
| How to cite this article: Calistru AM, Baudrier T, Gon�alves L, Azevedo F. Thrombophilia in venous leg ulcers: A comparative study in early and later onset. Indian J Dermatol Venereol Leprol 2012;78:406 |
Abstract
Background: Several studies found that the patients with chronic venous ulcers (CVU) have an increased prevalence of thrombophilia (44-75%), similar to that observed in deep vein thrombosis (DVT). The patients who develop CVU before their 50 th birthday appear to represent a distinct group in terms of etiology, natural history and prognosis. Aim: To analyze the nature and prevalence of thrombophilia in patients with early onset of CVU (before 50-years old) compared with a group of patients with later onset. Methods: Twenty-seven consecutive patients of each group were studied . They underwent clinical assessment and blood testing for factor V Leiden, prothrombin G20210A, methyltetrahydrofolate reductase C677T, plasminogen activator inhibitor type 1 (PAI-1) mutations, antithrombin, proteins C and S levels, and also antiphospholipid antibodies (anticardiolipin antibodies and lupus anticoagulant), cryoglobulins and cryoagglutinins. Results: All the patients had at least one thrombophilia. The prevalences of single, 2 and ≥3 thrombophilias were 29.6%, 40.7% and 29.6%, respectively, in the early onset group, compared with 33.3%, 59.2% and 7.4% in the later onset group. The PAI-1 4G/4G homozygous mutation was significantly more common in patients with early onset of ulcer. The prevalences of factor V Leiden, prothrombin G20210A, elevated titer of antiphospholipid antibodies and the presence of cryoglobulins were higher in the early onset group, although the differences were not statistically significant. Conclusion : Our study brings evidence of a higher thrombophilic risk among the patients with early onset of the CVU as they had significantly higher prevalence of multiple (≥3) thrombophilias (P=0.03), homozygous mutations (P=0.03) and family history of leg ulcer (P=0.02) when compared with patients with later onset. Thrombophilia screening is important in patients with CVU before the age of 50 in order to stratify the thrombotic risk and to allow an appropriate prophylactic and therapeutic management.Introduction
Chronic venous ulcers (CVU) affect 1% of the adult population. They are associated with a marked reduction in the quality of life [1] and consume 1-2% of the healthcare spending in most developed countries. [2]
Thrombophilia consists of the predisposition to form clots inappropriately with consecutive venous thromboembolic events. It results from inherited or acquired factors or, more commonly, from their interaction. [3] Inherited hypercoagulable factors include antithrombin, protein C, protein S deficiencies, factor V Leiden (resulting in activated protein C resistance - APCR), prothrombin G20210A [3] and plasminogen activator inhibitor type 1 (PAI-1) mutations. [4] Hyperhomocystinemia may be both an acquired and inherited abnormality, the most common form of genetic hyperhomocystinemia resulting from methyltetrahydrofolate reductase (MTHFR) C677T mutation. [3] Acquired factors include antiphospholipid antibodies, immobility, trauma, postoperative state and malignancy. [5] Cryoglobulins and cryoagglutinins are rare acquired abnormalities that may be associated with small vessel thrombosis. [6]
Hypercoagulable disorders may cause ulceration as a consequence of postphlebitic syndrome following clinical and subclinical deep venous thrombosis (DVT), associated with microvascular thrombosis and the development of pericapillary fibrin cuff. [7] Several studies found that the patients with CVU have an increased prevalence of thrombophilia (44-75%), similar to that observed in patients with DVT. [8],[9],[10]
Most of CVU appear after the age of 65. MacKenzie et al., observed that the patients who develop CVU before their 50 th birthday appear to represent a distinct group in terms of etiology, natural history and prognosis. [11] However, data comparing thrombophilia in early and late onset of CVU is lacking. The aim of this study is to analyze the nature and prevalence of thrombophilia in patients with early onset of CVU (before 50-years old) compared with a group of patients with later onset of CVU (after the age of 50).
Methods
Consecutive patients with CVU observed at our Dermatology Department during a period of 13 months (June 2010-June 2011) were studied. Venous etiology of the ulcers was established on clinical grounds. All the patients had at least two of the following clinical features: Leg edema (51 patients), noticeable varicose veins (48 patients), hemosiderosis (25 patients), lipodermatosclerosis (11 patients), stasis eczema (10 patients) and atrophiae blanche (10 patients). The ulcers were located in the medial malleolar portion of the leg in 47 patients, with upward extension to the lower half of the leg in nine of them. Seven patients had their ulceration located in the external malleolar or anterior and lateral regions of the leg.
Patients who were undergoing anticoagulation therapy were excluded due to the known interference with thrombophilia testing. We also excluded patients with ulcers of other etiologies, such as diabetes (fasting plasma glucose ≥126 mg/dl) and arterial insufficiency, on base of positive history of intermittent claudication, ischemic cardiopathy or stroke, diminished or absent popliteal, posterior tibial and dorsalis pedis arteries pulses, unsatisfactory distal perfusion with capillary nail refill time ≥3 seconds and positive Buerger test. Doppler study was not performed as clinical evaluation was considered sufficient to exclude significant peripheral arterial disease. Patients with leg ulcers caused by other disorders such as vasculitis, neoplasm, infection or pyoderma gangrenosum, diagnosed with biopsy, were excluded too. Twenty-seven patients with onset of the CVU before the age of 50 were included in the early onset group and 27 patients with onset of the CVU after the age of 50 formed the later onset group. History and clinical assessment was performed and blood was drawn for determination of inherited and acquired thrombophilia. Note was made of age, sex, history of venous thromboembolic event (VTE), history of precipitant factors for thrombosis (smoking, surgery, lower limb fracture, prolonged immobilization, malignancy) and history of a first-degree relative with leg ulcer. The patients underwent blood testing for APCR, factor V Leiden when APCR was less than 0.84 (N>0.84), prothrombin G20210A, MTHFR and PAI-1 mutations, antithrombin, proteins C and S levels, and also antiphospholipid antibodies (anticardiolipin antibodies IgM and IgG, anti-β2 -glycoprotein antibodies and lupus anticoagulant), cryoglobulins and cryoagglutinins. Homocysteine levels were tested only in the first 25 patients included (13 from the early and 12 from the later onset group), as the test was no longer available at our laboratory.
Antithrombin activity and protein C were measured using chromogenic assays (STA Antithrombin III and STA Protein C chromogen - DIAGNOSTICA STAGO, Roche Diagnostics® ), with reference ranges of 80%-120% and 70-130%, respectively. A clotting assay was used to test protein S (STA Protein S Clotting - DIAGNOSTICA STAGO), with a normal range of 50-120% in female and 60-120% in male patients. Activated protein C resistance was determined with a clotting assay (APC Resistance - COATEST APC Resistance V - CHROMOGENIX® ), with a normal range >0.84 (normalized ratio). Prothrombin G20210A Kit and Factor V Leiden Kit, both from Roche Diagnostics® , and a LightCycler 2.0 instrument, were used to assess the two mutations. MTHFR C677T and PAI-1 mutations were determined using Lightcycler-DNA Master Hybridization probes from Roche Diagnostics® and primers and probes synthesized by TIB Molbiol® . The anticardiolipin and anti-β2 -glycoprotein 1 antibodies IgM and IgG were detected using a MICRO-ELISA immunoenzymatic assay (QUANTA Lite TM ACA IgM and IgG and QUANTA Lite TM β2 GPI IgM and IgG, INOVA Diagnostics, Inc.). Pathological values are: IgG or IgM>15 GPL-U/ml. Lupus anticoagulant was assessed using two clotting tests, both from American Diagnostica Inc.: DVVtest and DVV confirm. The lupus anticoagulant status was defined in relation to the normalized ratio (NR) between the two tests, as follows: Negative (NR<1.2), weakly present (1.2-1.5) moderately present (1.5-2) and strongly present (>2). The cryoglobulins were detected by a serum precipitation reaction. It was considered positive when precipitation occurred in a sample stored at 4°C, while the control sample stored at room temperature showed no reaction. The detection and titration of cryoagglutinins was performed according to the methods described in the Technical Manual of American Association of Blood Banks. [12] A titer of greater than 1:64 was considered abnormal. Homocysteine was measured using an immunoassay from Abbot Laboratories, with a normal range of 5.0-15.0 μmol/L.
Information about the nature of the tests was given, using the local language. Consent was obtained from all of the patients, being witnessed by the first two authors. They were told about the possible usefulness of the tests in explaining the development of the leg ulcers, but that results might not affect their subsequent treatment. The tests were performed and supported by the hospital, as being part of the procedure for our patients, when needed.
Statistical analysis of the data was performed using the SPSS19 software. The χ2 -test was used to determine the two-tailed statistical significance of differences between proportions in 2 × 2 tables (categorical variables). A P value of less than 0.05 was considered significant.
Results
There were 27 patients in the early onset group (12 men and 15 women), with a mean actual age of 57.6 years (range 23-81) and 27 patients in the later onset group (7 men and 20 women) with a mean actual age of 70 years (range 55-84). The average age of ulcer onset was of 36 years (range 18-49) in the early and of 62 years (average 50-77) in the later onset group.
All the patients had at least one thrombophilia. The prevalence of single, 2 and ≥3 thrombophilias were 29.6%, 40.7% and 29.6%, respectively in the early onset group, compared with 33.3%, 59.2% and 7.4% in the later onset group [Figure - 1]. The early onset group had significantly more patients with ≥3 thrombophilias (n=8) than the control group (n=2) (P=0.03). The total number of homozygous mutations (including PAI-1 4G/4G genotype, MTHFR and prothrombin G20210A mutations) was significantly higher in the early onset group (n=19) compared with the other group (n=11) (P=0.03).
 |
| Figure 1: Prevalence of thrombophilia in patients with onset of chronic leg ulcer before 50 years old (cases) compared patients with onset of CLU after the age of 50 (controls) |
The individual thrombophilia data are summarized in the [Table - 1] below. The prevalences of factor V Leiden, prothrombin G20210A, elevated titer of antiphospholipid antibodies and the presence of cryoglobulins were higher in the early onset group, while the overall prevalences of MTHFR mutation and PAI-1 4G allele (including heterozygotes and homozygotes) were higher in the later onset group, although the differences were not statistically significant. Specifically, MTHFR mutation was found in 16 (59.2%) patients from the early onset group and in 17 (62.9%) in the later onset group; PAI-1 4G allele was found in 23 (85.1%) patients from the early onset group and in 26 (96.2%) in the later onset group. The PAI-1 4G/4G genotype was significantly more common in patients with onset of ulcer before the age of 50 (n=16) compared with later onset (n=8) (P=0.03).
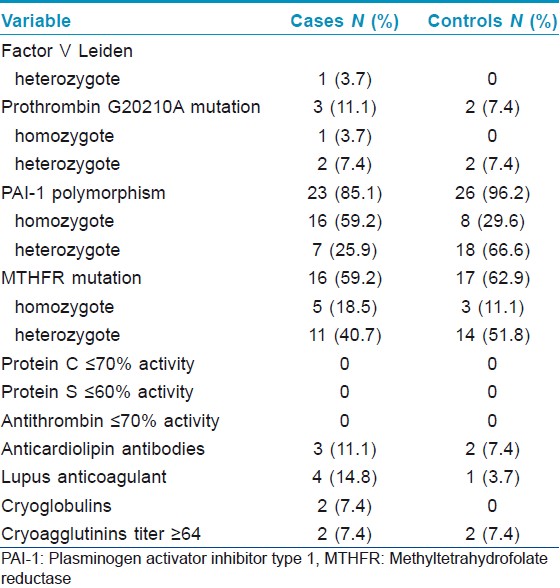
No cases of antithrombin, protein C and protein S deficiency were found in our series.
High levels of homocysteine (≥15 μmol/L) were found in 9 (36%) of the 25 patients tested (2 from the early and 7 from the later onset group) and eight of them had the MTHFR mutation (3 homozygotes and 5 heterozygotes).
History of CVU in a first-degree relative was found in 11 patients in the early onset group compared with only four patients from the other group and this difference was significant (P=0.02). History of DVT was present in eight patients from the early onset group (two of them with recurrent episodes) and six patients from the later onset group (one of them with recurrent episodes). Precipitant factors for thrombosis before the ulcer onset were identified in eight patients from the early onset group (three patients with lower limb fracture, three heavy smokers and two patients with prolonged immobilization) and three patients from the later onset group (lower limb fracture).
Discussion
Our study found that multiple (≥3) thrombophilias and homozygous mutations were significantly more common in patients with onset of CVU before the age of 50 comparing with later onset. When ≥2 factors were taken into consideration, no difference was found between early (19/27) and later onset (18/27) groups. Only the coexistence of ≥3 factors was significant.
Individuals with more than one thrombophilia (homozygotes or compound heterozygotes) have an increased risk of venous thromboembolic events (VTE). [13] Patients who develop venous leg ulceration before their 50 th birthday are more likely to have a history of DVT, [11] which, in turn, is recognized as a major risk factor for intractable chronic venous ulcers. [7] The higher tendency for a hypercoagulable state in the case group may explain the onset of ulceration earlier in life.
All our patients had at least one thrombophilia. The reported prevalence of at least one thrombophilia in CVU was of 41% [6] and 75% [7] in two studies not including the MTHFR and PAI-1 mutations and of 70% in another study which included MTHFR mutation but not the PAI-1 polymorphism. [10] The highest prevalence (100%) of at least one thrombophilia found in our study may be explained by the inclusion of the PAI-1 polymorphism and MTHFR mutation in the hypercoagulability testing.
PAI-1 is the central component of the fibrinolytic system. The 4G allele is associated with elevated levels of PAI-1, [14] which are higher in homozygotes than heterozygotes, [15] and appears to increase the risk of venous thrombosis, particularly in subjects with other genetic thrombophilic defects. [4] The prevalence in the general population was reported to be of 18.4% for the 4G/4G genotype and of 44.2% for the 4G/5G genotype. [15] In a population living in the same geographical region as ours (North of Portugal) the frequency of PAI-1 4G allele was of 76% in patients with a history of DVT before the age of 40 years and of 68% in healthy controls, this value being superior to other reports. [16] Our study has shown a very high prevalence of 4G allele in CVU patients (49 of 54 patients; 90.7%), and a significantly higher prevalence of homozygotes in patients with onset of CVU before the age of 50 years compared with later onset.
The C677T polymorphism of the MTHFR gene is relatively common in general population. About 34-37% of the US Caucasians are heterozygous for this variant and 12% are homozygous. [13] It has been associated with slight elevations of homocysteine levels, especially when folate deficiency is present; hyperhomocysteinemia in turn increases the risk of VTE. [13] Our study revealed a prevalence of the C677T polymorphism of 61% in patients with CVU, higher than reported in general population, but no significant difference was found between the early and later onset groups.
Factor V Leiden is present in 5% of the white population and in 7.7-36% of the CVU patients; [9] in this study it was found in one patient with early onset of the CVU.
Prothrombin G20210A mutation is present in 2% of the white population and has been reported in 0-4% of the CVU patients. [9] One study performed in a population from the same geographical area of Portugal found higher prevalence of heterozygosity for the G20210A allele, namely of 5% in healthy population and of 12.5% in patients with a history of DVT before 40 years old. [17] In our study this mutation was present in five out of 54 patients with CVU (9.2%); 3 of them, including the only homozygote patient, had the onset of the ulcer before the age of 50.
Antithrombin, protein C and protein S deficiencies, are established genetic factors for thrombophilia [3] and were found in percentages of 11%, 8% and 13%, respectively in a large cohort of CVU patients. [18] Our study did not reveal deficiencies of antithrombin, protein C and protein S, probably due to the smaller number of patients and regional particularities of the population. Another possible explanation is that patients carrying these defects may be under anticoagulation treatment and so excluded from the study.
The antiphospholipid antibodies are present in 10-20% of the population and have been reported in 5.6-59% of the CVU patients. [9] A large study of 310 CVU patients did not find significant difference concerning the antiphospholipid antibodies between the groups with or without post-thrombotic syndrome. [18] Ten out of 54 patients (18.5%) in the present series had elevated anticardiolipin antibodies or positive lupus anticoagulant, most of them (7) having the onset of the ulcer before 50 years old.
Cryoglobulins and cryoagglutinins are immunoglobulins that precipitate at cold temperatures and may cause vaso-occlusive phenomena. They constitute rare causes of leg ulceration. [19],[20] Two patients in the early onset group had positive cryoglobulins, but none in the later onset group, while cryoagglutinins were found in two patients of each group.
The presence of a single heterozygote thrombophilia does not confer a significant increase in the risk of thrombosis, but when more such abnormalities coexist and are accompanied by acquired thrombophilia and precipitant factors, then the risk of DVT increases dramatically. [7] These patients should avoid smoking or oral contraceptive, may receive prophylactic hypocoagulation when precipitant factors are added (surgery, immobilization), or may be candidates for prolonged hypocoagulation after a VTE. [13]
Regarding the management of the CVU in patients with thrombophilia, several reports have found beneficial effects of warfarin, dalteparin, clopidogrel, aspirin, or combination aspirin-pentoxyphiline. [9],[21],[22]
Conclusions
Patients with onset of the CVU before the age of 50 have significantly higher prevalence of multiple (≥3) thrombophilias, homozygous mutations and family history of leg ulcer when compared with patients with later onset. The PAI-1 mutation, which was not tested before in CVU patients, had a significantly higher prevalence of homozygotes in patients with early onset. Our data bring new evidence of a higher thrombophilic risk in the patients with early onset of CVU, supporting the hypothesis that these patients represent a distinct group. Thrombophilia screening is important in patients with CVU before the age of 50 in order to stratify the thrombotic risk and to allow an appropriate prophylactic and therapeutic management. Future studies will establish the paper of antiaggregant or anticoagulant therapy in CVU associated with thrombophilia.
| 1. |
Reichenberg J, Davis M. Venous ulcers. Semin Cutan Med Surg 2005;24:216-26.
[Google Scholar]
|
| 2. |
Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: Incidence and prevalence in the elderly. J Am Acad Dermatol 2002;46:381-6.
[Google Scholar]
|
| 3. |
Khan S, Dickerman JD. Hereditary thrombophilia. Thromb J 2006;4:15.
[Google Scholar]
|
| 4. |
Tsantes AE, Nikolopoulos GK, Bagos PG, Rapti E, Mantzios G, Kapsimali V , et al. Association between the plasminogen activator inhibitor-1 4G/5G polymorphism and venous thrombosis. A meta-analysis. Thromb Haemost 2007;97:907-13.
[Google Scholar]
|
| 5. |
Barger AP, Hurley R. Evaluation of the hypercoagulable state: Whom to screen, how to test and treat. Postgrad Med J 2000;108:59-66.
[Google Scholar]
|
| 6. |
Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: Clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol 1991;25:21-7.
[Google Scholar]
|
| 7. |
Bradbury AW, MacKenzie RK, Burns P, Fegan C. Thrombophilia and chronic venous ulceration. Eur J Vasc Endovasc Surg 2002;24:97-104.
[Google Scholar]
|
| 8. |
Mackenzie RK, Ludlam CA, Ruckley CV, Allan PL, Burns P, Bradbury AW. The prevalence of thrombophilia in patients with chronic venous leg ulceration. J Vasc Surg 2002;35:718-22.
[Google Scholar]
|
| 9. |
Darvall KA, Sam RC, Adam DJ, Silverman SH, Fegan CD, Bradbury AW. Higher prevalence of thrombophilia in patients with varicose veins and venous ulcers than controls. J Vasc Surg 2009;49:1235-41.
[Google Scholar]
|
| 10. |
Brandt HR, de Lorenzo Messina MC, Hirayama JT, Belda W Jr, Benabou JE, Criado PR. Prevalence of thrombophilia associated with leg ulcers. Br J Dermatol 2009;160:202-3.
[Google Scholar]
|
| 11. |
MacKenzie RK, Brown DA, Allan PL, Bradbury AW, Ruckley CV. A comparison of patients who developed venous leg ulceration before and after their 50 th birthday. Eur J Vasc Endovasc Surg 2003;26:176-8.
[Google Scholar]
|
| 12. |
Roback J, Combs MR, Grossman B, Hillyer C, editors. Technical Manual of the American Association of Blood Banks. 16 th ed. United States: AABB; 2008.
[Google Scholar]
|
| 13. |
Varga EA, Kerlin BA, Wurster MW. Social and ethical controversies in thrombophilia testing and update on genetic risk factors for venous thromboembolism. Semin Thromb Hemost 2008;34:549-61.
[Google Scholar]
|
| 14. |
Tsantes AE, Nikolopoulos GK, Bagos PG, Bonovas S, Kopterides P, Vaiopoulos G. The effect of the plasminogen activator inhibitor-1 4G/5G polymorphism on the thrombotic risk. Thromb Res 2008;122:736-42.
[Google Scholar]
|
| 15. |
Festa A, D'Agostino R Jr, Rich SS, Jenny NS, Tracy RP, Haffner SM. Promoter (4G/5G) plasminogen activator inhibitor-1 genotype and plasminogen activator inhibitor-1 levels in blacks, Hispanics, and non-hispanic whites: The Insulin Resistance Atherosclerosis Study. Circulation 2003;107:2422-7.
[Google Scholar]
|
| 16. |
Mansilha A, Araújo F, Severo M, Sampaio SM, Toledo T, Henriques I, et al. The association between the 4G/5G polymorphism in the promoter of the plasminogen activator inhibitor-1 gene and deep venous thrombosis in young people. Phlebology 2005;20:48-52.
[Google Scholar]
|
| 17. |
Mansilha A, Araújo F, Sampaio S, Cunha Ribeiro LM, Braga A. The PORtromb Project: Prothrombin G20210A mutation and venous thromboembolism in young people. Cardiovasc Surg 2002;10:45-8.
[Google Scholar]
|
| 18. |
Zutt M, Krüger U, Rosenberger A, Schön MP, Neumann C, von Ahsen N, et al. Thrombophilia in patients with chronic venous leg ulcers-a study on patients with or without post-thrombotic syndrome. J Eur Acad Dermatol Venereol 2011;25:1432-9.
[Google Scholar]
|
| 19. |
Auzerie V, Chiali A, Bussel A, Brouet JC, Fermand JP, Dubertret L, et al. Leg ulcers associated with cryoglobulinemia: Clinical study of 15 patients and response to treatment. Arch Dermatol 2003;139:391-3.
[Google Scholar]
|
| 20. |
Mekkes JR, Loots MA, Van Der Wal AC, Bos JD. Causes, investigation and treatment of leg ulceration. Br J Dermatol 2003;148:388-401.
[Google Scholar]
|
| 21. |
Ibbotson SH, Layton AM, Davies JA, Goodfield MJ. The effect of aspirin on haemostatic activity in the treatment of chronic venous leg ulceration. Br J Dermatol 1995;132:422-6.
[Google Scholar]
|
| 22. |
Calistru AM, Cruz MJ, Baudrier T, Gonçalves L, Azevedo F. Leg ulcers revealing familial thrombophilia: Prothrombin G20210A gene mutation and plasminogen activator inhibitor type 1 (PAI-1) polymorphism. Eur J Dermatol 2011;21:414-5.
[Google Scholar]
|
Fulltext Views
3,073
PDF downloads
2,999





