Translate this page into:
Topical permethrin and oral ivermectin in the management of scabies: A prospective, randomized, double blind, controlled study
Correspondence Address:
Archana Singal
B-14, Law Apartments, Karkardooma, Delhi - 110 092
India
| How to cite this article: Sharma R, Singal A. Topical permethrin and oral ivermectin in the management of scabies: A prospective, randomized, double blind, controlled study. Indian J Dermatol Venereol Leprol 2011;77:581-586 |
Abstract
Background: Scabies is a highly contagious and intensely pruritic parasitic infestation. It is a re-emerging infection in the new millennium especially with HIV pandemic and a significant health problem in developing countries. Various treatment modalities have been used since time immemorial but the search for an ideal scabicide is ongoing. Aims: In this study, we compared the therapeutic efficacy of single application of topical 5% permethrin with oral ivermectin (200 μg/kg/dose) in a single-dose and a two-dose regimen in patients with scabies. Methods: 120 clinically diagnosed cases of scabies (>5 years of age and/or >15 kg) were randomized into three treatment groups A, B, C of 40 patients each; receiving either topical 5% permethrin (group A) or oral ivermectin (200 μg/kg/dose) in a single dose (group B) or double dose regimen (group C) repeated at 2 weeks interval. Patients were followed up at 1, 2, and 4 weeks interval. At each visit, cure rate (>50% improvement in lesion count and pruritus and negative microscopy) was assessed and compared. Results: Cure rate in three treatment groups at the end of 4 weeks was 94.7% (A), 90% (B), 89.7%(C), and thus all three treatment modalities were equally efficacious. However, at 1 week follow up, group A patients reported better improvement in both lesion count and pruritus. Conclusions: Both permethrin and ivermectin in both single and two dose regimen are equally efficacious and well tolerated in scabies. However, permethrin has a rapid onset of action.Introduction
Scabies is a highly contagious; intensely pruritic dermatosis caused by the mite Sarcoptes scabiei var hominis, an obligate human parasite and is transmitted by close, skin to skin contact. [1] Various treatment modalities have been used but the search for an ideal scabicide is ongoing. An ideal scabicide should be effective against adult mite and eggs, easily applicable or orally administered, non-sensitizing, non-irritating, non-toxic, economical, and safe in all age groups. [2] The mainstay of therapy in the present era is topical, and includes benzyl benzoate, crotamiton, gamma benzene hexachloride (lindane), and permethrin. [3] In addition, oral anti-parasitic agent ivermectin 200 μg/kg has been found to be an effective scabicidal agent as a single or two dose regimens given at 2 weeks interval. [4]
Permethrin 5% cream has been found to be more effective as compared to lindane and is FDA approved for the treatment of scabies. [5],[6] In developing countries like India where the disease is largely prevalent in the lower socioeconomic strata, acquiring permethrin is considered an expensive option. Besides, whole body application for hours together is inconvenient and leads to poor compliance. Ivermectin is administered orally and has the advantage of being cheaper thus improving the compliance. Ivermectin has been reported to be equally [7] or more [5] efficacious than lindane. However, a recent study revealed higher treatment failure with single-dose ivermectin than with benzyl benzoate. [8]
There is a paucity of high-quality studies that compare the various therapies for scabies. [9] Treatment efficacy of topical 5% permethrin and oral ivermectin has been compared in an open study from South India. [4] Another Indian study by Bachewar et al., [10] has compared benzyl benzoate, ivermectin and permethrin in an open label trial. [10] The present double-blind randomized study was undertaken to evaluate and compare the efficacy and safety of topical 5% permethrin with ivermectin in single and two dose regimens in the treatment of scabies.
Methods
The study was assessed and approved by the institutional ethics committee. A total of 120 consecutive patients with scabies over 5 years of age attending the dermatology outpatient clinic of Guru Teg Bahaudur hospital from December 2006 to March 2008 were included in the study. The diagnosis of scabies was made by the demonstration of eggs, larva, mites/mite products or fecal pellets by light microscopy in the scrapings from multiple representative or suspected skin lesions in 10% KOH and/or the presence of at least three of the following clinical criteria (a) demonstration of burrow; (b) presence of scabetic lesions at the classical sites; (c) nocturnal pruritus; (d) family history of similar illness.
Pregnant and lactating women, patients with immunodeficiency or severe systemic disease or with heavily crusted or nodular lesions, secondary infection or eczematization and coexisting dermatological disease that could interfere with the diagnosis and subsequent monitoring of scabies were excluded. Patients with a history of treatment with anti-scabetic or topical steroid in the previous 4 weeks or with known hypersensitivity to the trial drugs were also excluded.
After obtaining an informed consent, eligible patients were randomized to one of the three treatment groups A, B, C according to computer-generated random numbers. Group A patients received topical 5% permethrin cream on day 1 and placebo tablets of vitamin B-complex on day 1 and day 15. Group B patients had topical placebo (cream base) on day 1 and oral ivermectin 200 μg/kg on day 1 and placebo tablet of vitamin B-complex on day 15. Group C patients were given topical placebo on day 1 and oral ivermectin 200 μg/kg on day 1 and day 15. The placebos were similar to the trial drugs in color, shape, size, and consistency and were dispensed by a trained staff nurse in identical pre-coded and numbered container. For all the three groups neither the investigator nor the patients were aware of the composition of drugs allocated and the code was revealed only after the completion of the study. The drugs were dispensed by a trained staff nurse in a pre-coded and numbered container. Patients were instructed to apply the medication all over the body below the neck at night. Tablet formulations were advised to be taken before breakfast. They were advised about the importance of treating the family contacts and prevention of fomite transmission by washing all infested clothes and bedding and drying them in the sun. They were further advised not to apply or ingest any other medications for this disease during the study period. No antihistaminic drugs were administered during the study period. All family contacts were provided with topical 5% permethrin cream for single overnight application, free of cost but were not included in the study.
At the initial visit, all the study patients underwent physical and cutaneous examination, details of which were recorded on a pre-designed Proforma. Weight of all the patients was assessed to determine the dose of ivermectin. Socio-economic status of the patients was calculated as per the modified Kuppuswamy scale. The severity of scabies was assessed by counting the number of lesions and assigned a score of 0-3, arranged as follows: 0 = Free of lesions (no scabies), 1= 10 or fewer lesions (mild), 2= 11- 49 lesions (moderate), 3= 50 or more lesions (severe). The subjective assessment of pruritus was done by the patient on an increasing scale of 0-3 as follows: 0 = No pruritus, 1= Mild, 2= Moderate, 3= Severe. The objective assessment of pruritus was done by the patient on a scale of 0 to 10 using visual analogue scale (VAS) score.
Patients in all the three groups were followed-up at 1, 2, and 4 weeks interval. At every visit, patients had to undergo both clinical and microbiologic assessment. Clinical assessment included count of the lesions and grading of pruritus both subjectively and objectively by the patient as described on the first visit. Microbiological assessment was done by taking scrapings from the representative skin lesions either burrow or scabetic papules from classical sites like finger webs, flexural aspect of wrist, and penile shaft in 10% KOH for demonstration of mites or their products by light microscopy. Any adverse events were also recorded. The treatment was considered effective if at the end of 4 weeks, there was improvement in the pruritus assessed by the visual analogue scale, clinical improvement in the skin lesions with no new lesions and absence of mites or their products on microscopy. Improvement was graded as: Mild = less than 50% reduction in number of lesions and pruritus, Moderate = more than or equal to 50% reduction in the number of lesions and pruritus, Good = complete clearance of the lesions and pruritus. Complete clinical cure was defined as reduction in both the number of lesions as well as the grade of pruritus by more than or equal to 50% (i.e. moderate and good improvement) and negative microscopy . Treatment was considered to be a failure if at the end of 4 weeks there was no improvement in the pruritus and skin lesions, there was appearance of new lesions or persistence of mites and their products on microscopy. Also patients with mild improvement (i.e.<50% improvement in pruritus VAS and lesion count) were also included in the criteria for treatment failure.
The percentage of improvement was compared between the groups A, B, and C using Chi-square test and Fisher′s exact test, followed by Post-hoc Tukey′s test and the level of significance was kept at 5. One way ANOVA was used to compare the mean of variables.
Results
One hundred and twenty cases of scabies were randomized in a double blind manner to receive either topical 5% permethrin (group A), single dose oral ivermectin on day 1 (group B), or two dose regimen of oral ivermectin on day 1 and at day 15 (group C). Baseline clinical parameters between the three groups were comparable [Table - 1]. All 120 patients were followed up at the end of first week, while 117 patients were followed up at week 2 and week 4. Two patients in group A and one patient in group C were lost to follow-up.
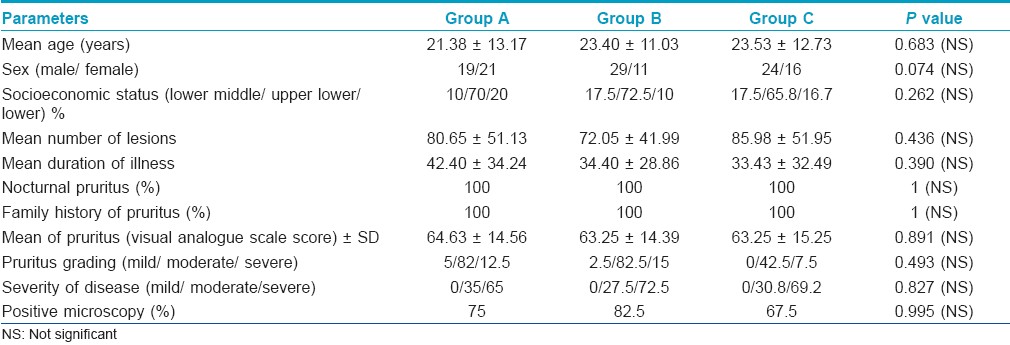
At first week follow-up, the decrease in number of lesions was maximum in group A observed in 37/40 (92.5%) patients followed by 29/40 (72.5%) patients each in groups B and C. Decrease in the number of lesions in group A was significantly more when compared to that of groups B and C (P=0.019). At 2 weeks, a total of 111/117 patients had clinical cure, of which 37/38 (97.4%), 38/40 (95%), 36/39 (92.3%) patients were in groups A, B, and C, respectively. At the end of 4 weeks, 110/117 (94%) patients had ≥50% improvement in the lesions (clinical cure), with 38/38 (100%), 36/40 (90%), and 36/39 (92.3%) patients being in groups A, B, and C, respectively. Therefore, at the end of 2 and 4 weeks, the outcome with respect to clinical cure in the three groups was not significantly different. 16 patients had complete clearance of lesions at 4 weeks, of which 10 were in group A and 6 in group C [Figure - 1].
 |
| Figure 1: Percentage improvement in lesions count |
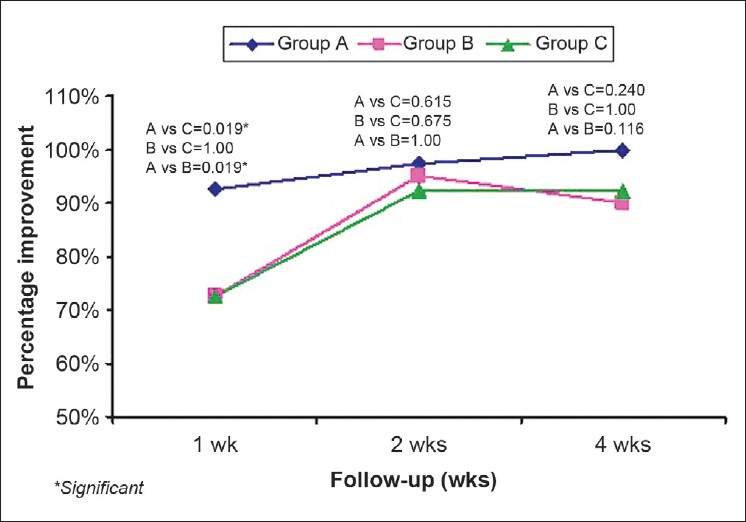
After the first week of treatment, the decrease in pruritus as assessed by VAS was maximum in group A in 27/40 (67.5%) patients as compared to 14/40 (35%) in group B and 12/40 (30%) in group C. The pruritus VAS score in group A was significantly lower as compared to group B and C (P=0.001 and 0.004), respectively. In the second week, a total of 90/117 patients had ≥50% improvement, with 33/38 (86.8%) patients in group A and 31/40 (77.5%), 26/39 (66.7%) patients in groups B and C, respectively. Decrease in pruritus was significantly more in group A as compared to that of group B (P value = 0.04). At the end of 4 th week, total 107/117 patients had ≥50% improvement with 36/38 (94.7%) patients in group A, 36/40 (90%) in group B, and 35/39 (89.7%) in group C. The difference was statistically not significant [Figure - 2].
 |
| Figure 2: Percentage improvement in pruritus (VAS) |
Ninety-four (94/120) patients had positive microscopy at first visit. At first post-treatment follow up, positive microscopy was observed in 22 patients (6/40=15% in group A and 8/40=20% each in groups B and C). At week 2, only 2/39 (5.1%) patients in group C demonstrated positive microscopy. The rest had complete microbiological clearance at weeks 2 and 4 [Figure - 3].
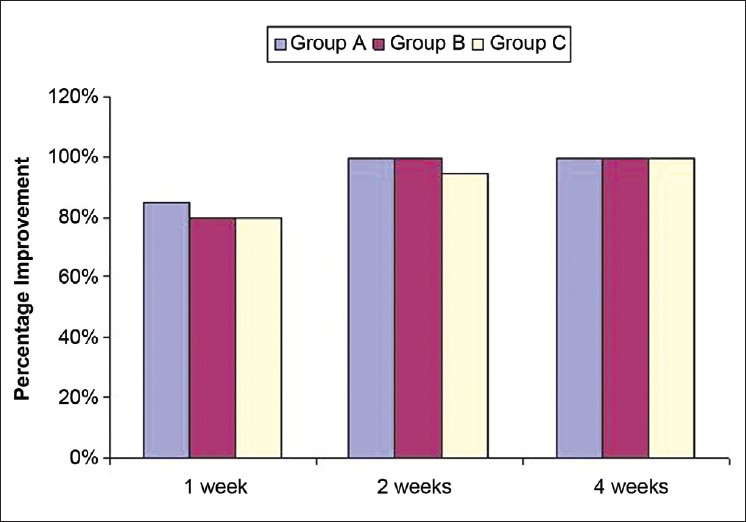 |
| Figure 3: Percentage improvement in microscopic clearance |
Complete clinical cure at first week of follow up was observed in 53/120 (44.2%) patients. Significantly more patients in group A, 27/40 (67.5%) patients, obtained complete clinical cure as compared to groups B and C 14/40 (35%) and 12/40 (30%). During the second week, 90/117 (76.9%) patients had complete clinical cure, which included 33/38 (86.8%), 31/40 (77.5%), and 26/39 (66.7%) patients respectively in groups A, B, and C. At 4 weeks post treatment, 107/117 (91.4%) patients reported complete clinical cure including 36/38 (94.7%) patients in group A and 36/40 (90%), and 35/39 (89.7%) patients in groups B and C, respectively. The difference was statistically insignificant [Figure - 4].
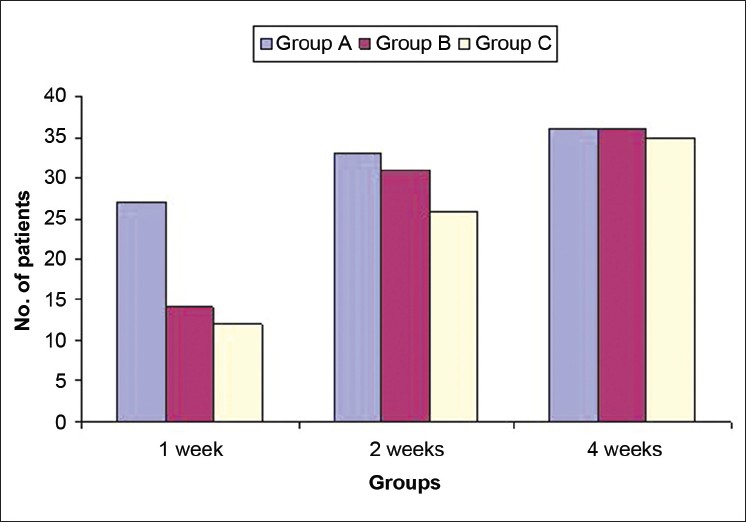 |
| Figure 4: Comparison of complete clinical cure in three groups A, B, and C |
Treatment failure was observed in a total of 10/117 (7.9%) patients at 4 weeks out of which 2 were in group A, 4 patients each in groups B and C. The difference was statistically not significant (P value= 0.769). These patients were switched over to the alternative therapy in the form of topical lindane.
Discussion
In this study, all the three treatment modalities revealed equal efficacy at the end of 4 weeks. However, topical 5% permethrin had shown faster improvement at first week follow up. Usha et al. [4] also observed superior efficacy with single application of 5% permethrin compared to single dose ivermectin (84.3% vs 50%) at 1 week in an open label study. Cure rate of 100% with oral ivermectin was observed at the end of 2 weeks in a study comparing ivermectin, permethrin, and benzyl benzoate. [10] Our study is the first randomized double-blind trial comparing the two, that is, relatively newer oral antiscabetic ivermectin in two dose regimen with the established topical 5% permethrin. Permethrin acts by disrupting the sodium channel current, resulting in delayed repolarization, paralysis, and death of the parasite. [11] Sodium channels are ubiquitous and therefore permethrin acts at all stages of the life cycle of the parasite. Furthermore, topical application ensures maximum concentration of the drug in the skin accounting for the superior efficacy. This study also concurs with the excellent cure rates (90% to 100%) observed in the initial studies with permethrin. Permethrin has been shown to be consistently the most effective scabicide with minimal toxicity and is currently the "gold standard" in scabies treatment.
Ivermectin acts by binding selectively and with high affinity to glutamate (or γ-amino butyric acid) gated chloride ion channels, which are present in invertebrate nerve and muscle cells, resulting in paralysis and death of the parasite. [12] Due to its specific site of action, ivermectin may not be effective against the younger stages of the parasite inside the egg because the nervous system has not yet developed. Furthermore, ivermectin may not adequately penetrate the thick egg shell. Probably ivermectin acts only at certain stages of life cycle of the parasite, as is reported in the case of onchocerciasis and strongyloidiasis. [12] Following oral administration, the concentration achieved in the skin may also be variable. However, the efficacy of a single dose of ivermectin in the treatment of scabies, both in immunocompetent and immunocompromised patients, has been well documented and is reported to be 70% to 100% by various authors. [13],[14],[15],[16],[17]
Transient burning sensation was reported by three patients and pruritus by two patients following permethrin application. To date, the use of ivermectin to treat scabies has not been conclusively associated with any serious adverse effects. [18] Oral ivermectin resulted in headache and nausea in four and two patients respectively that subsided spontaneously without any active intervention. Burning/stinging sensation was reported in 9.9%, pruritus in 6.4%, erythema in 2.1%, pain in 1.7%, tingling in 0.9% patients by Schultz et al., [6] as side effects of permethrin, while Chouela et al., [7] reported hypotension, abdominal pain, and vomiting with ivermectin.
Topical permethrin being both ovicidal and miticidal, theoretically appears to be more efficacious. In our study, though the outcome was equivocal at 4 weeks post treatment, topical permethrin scored over ivermectin in providing statistically significant higher cure rates at 1 week post treatment. This may have an important bearing in a highly contagious disease like scabies where chain or risk of transmission of disease may be curbed at a relatively early stage.
| 1. |
Sterling GB, Janniger CK, Kihiczak G. Neonatal scabies. Cutis 1990;45:229-31.
[Google Scholar]
|
| 2. |
Karthikeyan K. Treatment of scabies: Newer perspectives. Postgrad Med J 2005;81:7-11.
[Google Scholar]
|
| 3. |
Roos TC, Alam M, Roos S, Merk HF, Bickers DR. Pharmacotherapy of ectoparasitic infections. Drugs 2001;61:1067-88.
[Google Scholar]
|
| 4. |
Usha V, Gopalakrishnan NT. A comparative study of oral ivermectin and topical permethrin cream in the treatment of scabies. J Am Acad Dermatol 2000;42:236-40.
[Google Scholar]
|
| 5. |
Zargari O, Golchai J, Sobhani A, Dehpour AR, Sadr-Ashkevari S, Alizadeh N, et al. Comparison of the efficacy of topical 1% lindane vs 5% permethrin in scabies: A randomized, double-blind study. Indian J Dermatol Venerol Leprol 2006;72:33-6.
[Google Scholar]
|
| 6. |
Schultz MW, Gomez M, Hansen RC, Mills J, Menter A, Rodgers H, et al. Comparative study of 5% permethrin cream and 1% lindane lotion for the treatment of scabies. Arch Dermatol 1990;126:167-70.
[Google Scholar]
|
| 7. |
Chouela EN, Abeldano AM, Pellerano G, La Forgia M, Paple RM, Garsd A, et al. Equivalent therapeutic efficacy and safety of ivermectin and lindane in the treatment of human scabies. Arch Dermatol 1999;135:651-4.
[Google Scholar]
|
| 8. |
Ly F, Caumes E, Ndaw CA, Ndiaye B, Mahé A. Ivermectin verses benzyl benzoate applied once or twice to treat human scabies in Dakar, Senegal: A randomized controlled trial. Bull World Health Organ 2009;87:424-30
[Google Scholar]
|
| 9. |
Strong M, Johnstone PW. Interventions for treating scabies. Cochrane Database Syst Rev 2007;3:1-43.
[Google Scholar]
|
| 10. |
Bachewar NP, Thawani VR, Mali SN, Gharpure KJ, Shingade VP, Dakhale GN. Comparison of safety, efficacy, and cost effectiveness of benzyl benzoate, permethrin, and ivermectin in patients of scabies. Indian J Pharmacol 2009;41:9-14.
[Google Scholar]
|
| 11. |
Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med 2010;362:717-25.
[Google Scholar]
|
| 12. |
Campbell WC. Ivermectin, an antiparasitic agent. Med Res Rev 1993;13:61-79.
[Google Scholar]
|
| 13. |
Aubin F, Humbert P. Ivermectin for crusted (Norwegian) scabies. N Engl J Med 1995;332:612.
[Google Scholar]
|
| 14. |
Dunne CL, Malone CJ, Whitworth JA. A field study of the effects of ivermectin on ectoparasites of man. Trans R Soc Trop Med Hyg 1991;85:550-1.
[Google Scholar]
|
| 15. |
Glaziou P, Cartel JL, Alzieu P, Briot C, Moulia-Pelat JP, Martin PM. Comparison of ivermectin and benzyl benzoate for the treatment of scabies. Trop Med Parasitol 1993;44:331-2.
[Google Scholar]
|
| 16. |
Kar SK, Mania J, Patnaik S. The use of ivermectin in scabies. Natl Med J India 1994;7:15-6.
[Google Scholar]
|
| 17. |
Barkwell R, Shields S. Deaths associated with ivermectin in treatment of scabies. Lancet 1997;349:1144-5.
[Google Scholar]
|
| 18. |
Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, et al. Safety, tolerability and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol 2002;42:1122-33.
[Google Scholar]
|
Fulltext Views
9,404
PDF downloads
3,068





