Translate this page into:
Treatment of recalcitrant paediatric prurigo nodularis with tofacitinib, an exquisite example of bench-to-bedside translation of JAK-STAT expression
Corresponding author: Dr. Kabir Sardana, Department of Dermatology, ABVIMS & Dr. Ram Manohar Lohia Hospital, Baba Kharak Singh Rd, Gurudwara Bangla Sahib, Connaught Place, Delhi, New Delhi, India. kabirijdvl@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sardana K, Mathachan SR, Agrawal D. Treatment of recalcitrant paediatric prurigo nodularis with tofacitinib, an exquisite example of bench-to-bedside translation of JAK-STAT expression. Indian J Dermatol Venereol Leprol. 2024;90:238–40. doi: 10.25259/IJDVL_362_2023.
Dear Editor,
Prurigo nodularis is characterised by hyperkeratotic, eroded papulonodular lesions, distributed symmetrically on the extensors and trunk, often triggered by intense pruritus lasting more than 6 weeks, often restricted to middle-aged and older persons (5th decade), and rarely in children. Most therapies have high failure rates evident by the varied agents administered, including pregabalin, gabapentin, cyclosporine, methotrexate and thalidomide.1 Given the diverse array of mechanism involved in the pathogenesis of paediatric prurigo nodularis, a combination of agents is needed for effective treatment. Although tofacitinib, a non-selective Janus kinase (JAK) inhibitor, has been utilised in adult prurigo nodularis cases, there is currently limited translational data available regarding its use, specifically in relation to JAK-STAT expression in paediatric prurigo nodularis.2
A 13-year-old non-atopic boy, presented to the dermatology outpatient department of ABVIMS and Dr. Ram Manohar Lohia Hospital, New Delhi, with generalised excoriated papulonodular lesions on both upper and lower limbs (extensors more than flexors) and trunk for four years [Figure 1]. Skin biopsy revealed compact orthokeratosis with focal parakeratosis, a prominent granular layer, and irregular acanthosis. The papillary dermis showed moderate lymphoplasmacytic infiltration and vertically oriented collagen bundles [Figure 2]. The baseline pruritus grading system score (PGSS) was 16 out of 19. Treatment history included the unsuccessful use of methotrexate, thalidomide, topical steroids, and antihistamines. A detailed investigative panel to rule out any secondary cause of prurigo nodularis [complete blood count, fasting sugar levels, liver and kidney function tests, thyroid function tests, viral markers (HIV-ELISA, hepatitis B and C serology)] was normal. Based on our previous study on STAT expression with a heightened expression of T-helper (Th2) cytokines and the published data on the use of tofacitinib in adult prurigo nodularis, we offered the parents tofacitinib.2 Baseline investigations, including a complete hemogram, liver and kidney function tests, Mantoux test, IGRA (Interferon-Gamma Release Assay), chest X-ray, fasting lipid profile, and viral markers, were done prior to the initiation of tofacitinib, which was started in a dose of 5 mg twice daily along with topical steroid and salicylic acid combination (clobetasol propionate 0.05% and salicylic acid 3%), emollients and antihistamines. There was a dramatic improvement in pruritus within one week after starting the therapy, and continued therapy led to the complete resolution of lesions in 3 months, after which the dose was reduced to 5 mg once daily and stopped after a month, with no adverse sequelae [Figure 3]. The patient achieved a PGSS score of 2 and is in remission for three months since the discontinuation of the medication.

- Multiple papulonodular lesions distributed on the trunk (baseline PGSS score-16, severe)
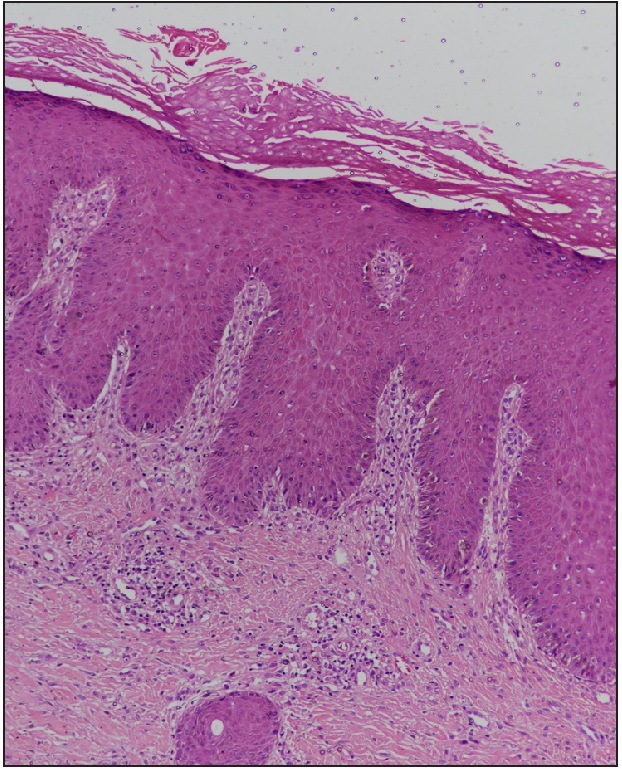
- Epidermis shows compact orthokeratosis with focal parakeratosis, prominent granular layer and irregular acanthosis. The papillary dermis shows moderate lymphoplasmacytic infiltrate and vertically oriented collagen bundles (H&E, 100x)
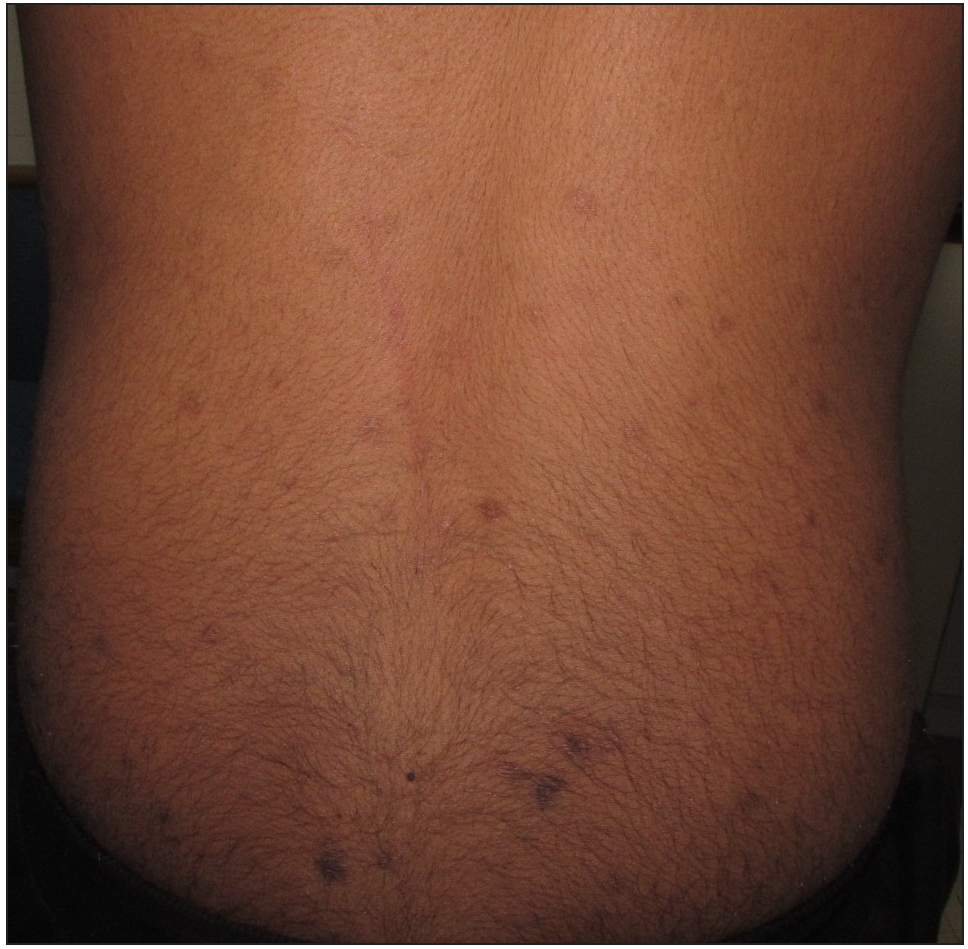
- Improvement in the lesions after 3 months of treatment with tofacitinib (post-treatment PGSS -2, mild)
The literature on prurigo nodularis in children is currently limited. However, in a study by Huang et al. in 2021, the estimated prevalence of paediatric prurigo nodularis was 21.6 cases per 100,000 individuals.3 The morphology of prurigo nodularis in children is characterised by hyperkeratotic, pruritic dome-shaped nodules, papules or plaques with hyperpigmented margins, similar to the presentation observed in adults. However, prurigo nodularis is more commonly associated with atopic dermatitis in children than in adults.3
The pathophysiology of prurigo nodularis is characterised by crosstalk between inflammatory cells, proinflammatory cytokines, neuropeptides and hyperplasia of cutaneous neurons, resulting in a vicious itch-scratch cycle.4 There is an increased expression of interleukin (IL) 4, IL 13, IL 31, IL22 and IL 17 in prurigo nodularis, which mediate their action via the JAK-STAT pathway. IL‐4α establishes a JAK1‐dependent, chronic itch cycle by stimulating sensory neurons. The predominant Th2 cytokines, IL-31 and IL-4, remain the major mediators that bridge the gap between immune cells, the nervous system, and the skin, and a systemic and cutaneous Th2 immune polarisation is characteristic of prurigo nodularis2,4 [Figure 4]. Our previous study highlighted the increased expression of STAT 3 and 6 in lesional skin biopsies of prurigo nodularis, which implied a role for Th2, Th17 and Th 22 cells in the pathogenesis of prurigo nodularis.2 Logically, a drug class that can inhibit multiple cytokines is needed, and thus instead of biologics like dupilumab and nemolizumab, which target IL4R α and IL31RA respectively, a JAK inhibitor drug would be ideal. Tofacitinib, a JAK 1, 3 inhibitor, would inhibit Th2 and Th17 expression and specifically inhibit the signalling of IL4 (JAK1, JAK3, STAT6) and IL31 (JAK1, STAT3, STAT5), making it a justifiable and cost-effective treatment option in prurigo nodularis1,2,5,6 [Figure 4]. We recommend a tapering approach for tofacitinib treatment once the clinical response has been achieved. Gradually reducing the dosage allows a smooth transition to other topical agents and antihistamines. In cases where relapse occurs upon discontinuation, a low dose of tofacitinib and other topical treatments can be continued. This combined approach, along with regular lab monitoring, can effectively manage symptoms and prevent relapse in these individuals. However, more data needs to be generated in the paediatric age group and the existent treatment options for prurigo nodularis are detailed in Table 1.1
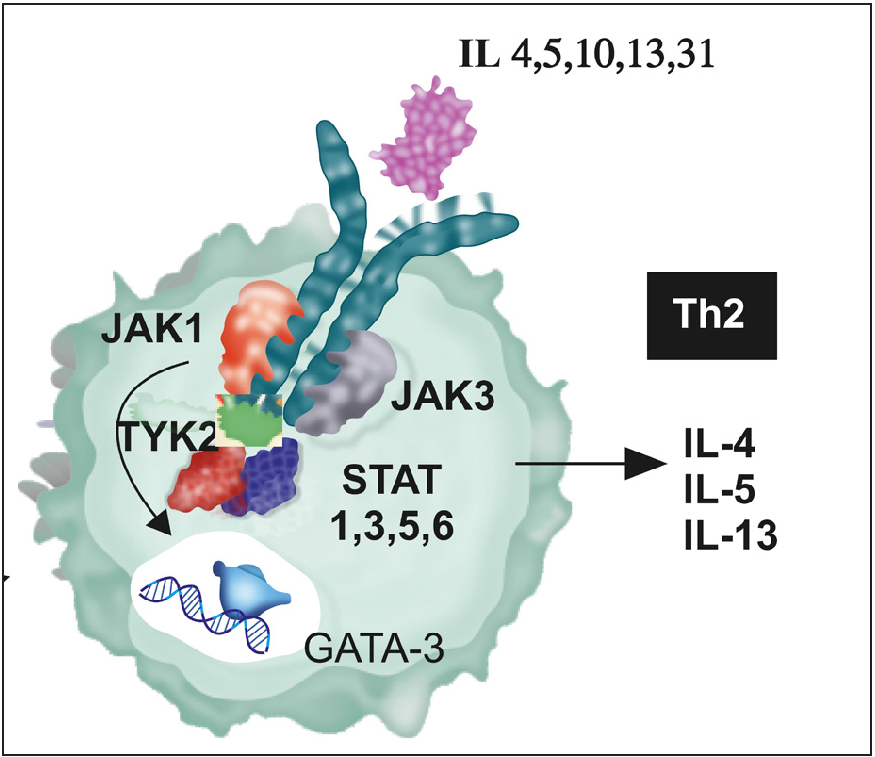
- The predominant mediators of prurigo nodularis are IL 4, 13, 31, which mediate their action via the T helper 2 cells, and the signal pathway is JAK1, 3 and STAT 1,3,5,6 which indicates a Th2 response. Published data have implicated STAT 6, and thus a JAKi drug that inhibits the signal pathway of Th2 cells would effectively abrogate the effect of the cytokines, tofacitinib is an ideal prototype JAKi for this action.
| Treatment | Neural targets | Immunological targets |
|---|---|---|
| 1st Line |
Topical capsaicin Topical ketamine/lidocaine |
Topical/intralesional corticosteroids Topical calcineurin inhibitors Topical calcipotriol |
| 2nd Line |
NK1receptor antagonist High dose gabapentinoids Antidepressants(SNRI, SSRI, TCA) |
Cyclosporine Methotrexate NBUVB/PUVA |
| 3rd Line | Thalidomide |
IL31 inhibitors Azathioprine Dupilumab |
| 4th line | Cannabinoids |
JAK inhibitors Mycophenolate mofetil |
SNRI: Serotonin and norepinephrine reuptake inhibitors, SSRI: Selective serotonin reuptake inhibitors, TCA: Tricyclic antidepressants, NBUVB: Narrowband ultraviolet-B, PUVA: psoralen plus ultraviolet-A radiation, IL: Interleukin, JAK: Janus kinase
Our case translates the tissue-based JAK-STAT expression in prurigo nodularis which is the signal pathway for cytokines and tofacitinib with its action on the implicated cytokines and the itch pathway would be a effective drug in recalcitrant cases of prurigo.1,5,6
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- A prospective study examining the effect of selected topical and systemic drugs on pruritus grading system score and STAT 6 expression in patients of prurigo nodularis. Indian J Dermatol. 2021;66:638-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A prospective study examining the expression of STAT 1, 3, 6 in prurigo nodularis lesions with its immunopathogenic and therapeutic implications. J Cosmet Dermatol. 2022;21:4009-15.
- [CrossRef] [PubMed] [Google Scholar]
- Real-world disease burden and comorbidities of pediatric prurigo nodularis. J Am Acad Dermatol. 2022;86:655-57.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of recalcitrant nodular prurigo with tofacitinib. Clin Exp Dermatol. 2020;45:918-20.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of prurigo nodularis with tofacitinib: The experience from a single center. Int J Dermatol. 2023;62:e293-e295.
- [CrossRef] [PubMed] [Google Scholar]





