Translate this page into:
Ulcerated lobular panniculitis: An unusual initial presentation of anti-Mi-2-alpha positive dermatomyositis
Corresponding author: Dr. Pravitha Pookkottil Balakrishnan, Junior Resident, Department of Dermatology, Venereology and Leprology, Government Medical College, Thrissur, Kerala, India. pravithabalakrishnan5@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ambooken B, Balakrishnan PP, Asokan N, Krishnan J. Ulcerated lobular panniculitis: An unusual initial presentation of anti-Mi-2-alpha positive dermatomyositis. Indian J Dermatol Venereol Leprol 2022;88:381-4.
Sir,
Atypical presentations of dermatomyositis often result in delayed diagnosis. We report a case of dermatomyositis which presented as ulcerative panniculitis.
A 30-year-old lady with no known comorbidities presented with non-productive cough and low-grade fever for two months. She had lost six kilograms of body weight during this period. One month later, she noticed a painful subcutaneous swelling on the left arm. There was no history of dysphagia or loss of appetite. General examination was normal. Respiratory system examination revealed decreased breath sounds in the left lower lung field with no added sounds. There was an ill-defined, tender, erythematous, indurated swelling on the left arm. Hemogram was normal except for a minimal elevation of erythrocyte sedimentation rate of 26 mm in the first hour (normal range: 0–7 mm in the first hour). Serum C-reactive protein and blood sugar levels were within normal limits. Hepatic, renal, and thyroid function tests were normal. The anti-nuclear antibody by immunofluorescence method was negative. Serology for human immunodeficiency virus, hepatitis B and hepatitis C viruses were negative. Chest X-ray and high-resolution computed tomography of thorax showed minimal pleural effusion on the right side and moderate pleural effusion on the left side. The lung parenchyma was normal and there was no mediastinal lymphadenopathy. Mantoux test was negative. Pleural fluid was exudative with elevated protein of 4.6 gm% (normal <1.5 gm%) and raised adenosine deaminase of 81 U/mL (<40 U/ml is not suggestive of tuberculosis). Cytological analysis of pleural fluid showed only lymphocytes and no atypical cells. Pleural fluid culture for bacteria and acid-fast bacilli yielded no growth. Ziehl-Neelsen staining and cartridge-based nucleic acid amplification test for acid-fast bacilli were negative on sputum, induced by nebulization with normal saline. Sputum culture was sterile. Ultrasonogram and high-resolution computed tomography of abdomen and pelvis were normal. The soft tissue ultrasonogram of the tender swelling on the left upper arm showed diffuse subcutaneous oedema on the medial and posterior aspects. Histopathology of this lesion was suggestive of lobular panniculitis with a lymphocyte-predominant infiltrate [Figures 1a and b]. As the patient was having low-grade fever, cough, loss of weight, exudative type of pleural effusion with predominant lymphocytes and a raised adenosine deaminase in pleural fluid, she was started on anti-tuberculosis therapy by her physician.
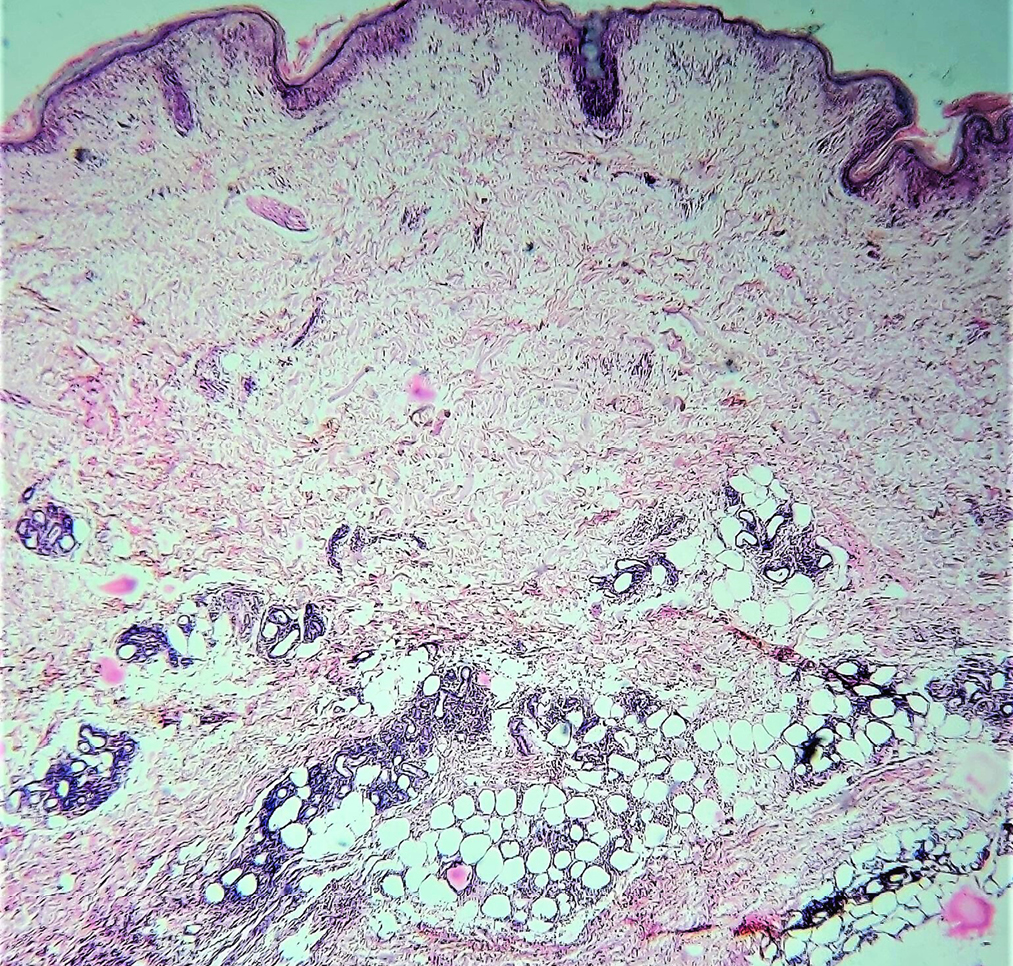
- Lobular panniculitis showing infiltrate around the subcutaneous fat cells (Haematoxylin and eosin, ×40)
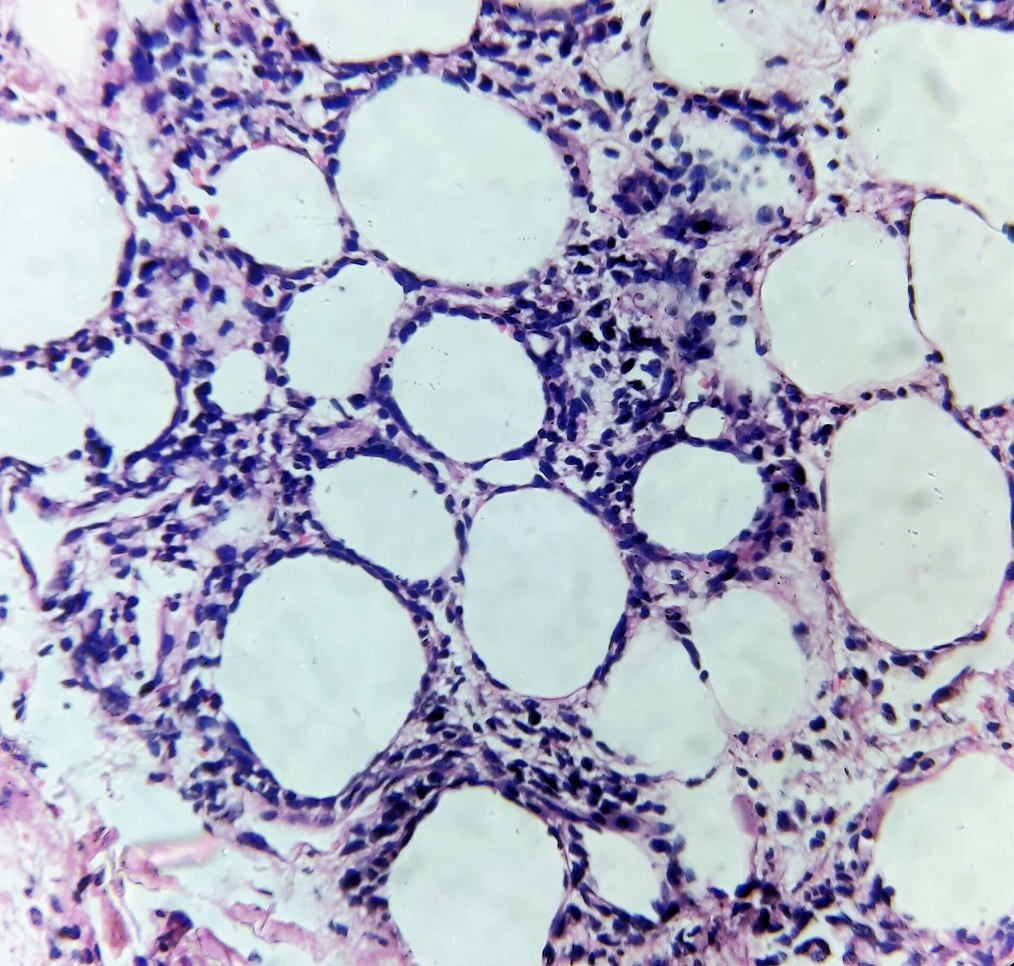
- Lobular panniculitis showing predominantly lymphocytic infiltrate around the subcutaneous fat cells (Haematoxylin and eosin, ×400)
After six weeks of anti-tuberculosis therapy, she presented with oedema of the face and extremities. She also developed painful ulcers on both hands, forearms and at the biopsy site. The fever was persisting. There was no history of photosensitivity. She also had difficulty in getting up from the squatting position and climbing stairs. General examination revealed pallor and bilateral pitting pedal oedema. There was bilateral periorbital erythema and oedema with a tender linear ulcer on the left infraorbital area [Figure 2a]. The tender subcutaneous swelling on the left arm showed induration and ulceration [Figure 2b]. She also had multiple painful ulcers on both elbows and sides of hands with erythematous plaques on both palms [Figure 2c]. Oral examination showed a painless eroded erythematous plaque on the hard palate [Figure 2d]. Motor system examination showed grade four power of thigh muscles. Clinical possibilities of dermatomyositis, systemic lupus erythematosus or overlap syndrome possibly exacerbated by anti-tuberculosis therapy were considered, and it was discontinued.
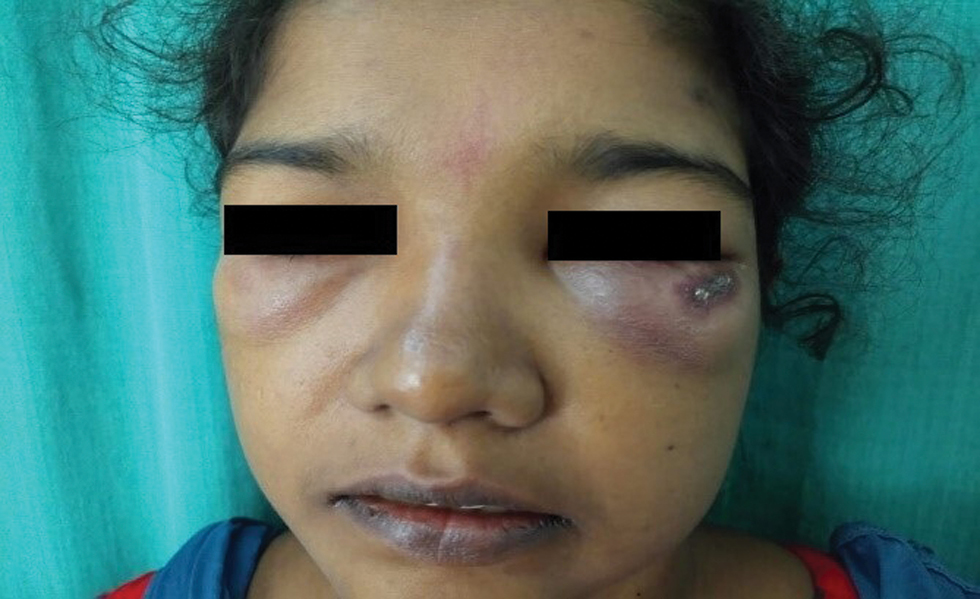
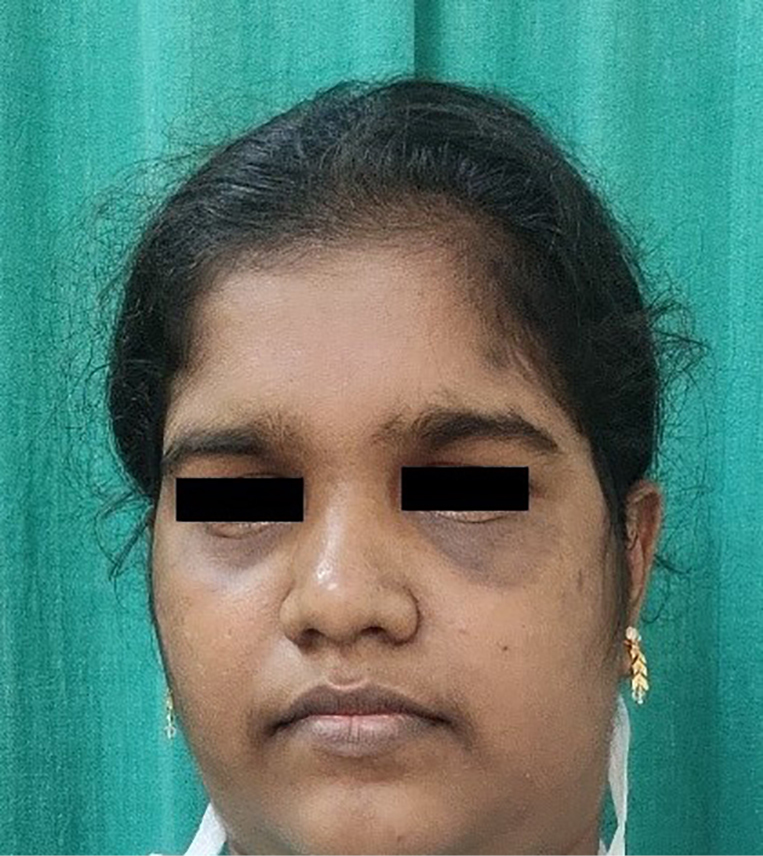
- Bilateral periorbital erythema and oedema with an ulcer on the left infraorbital area
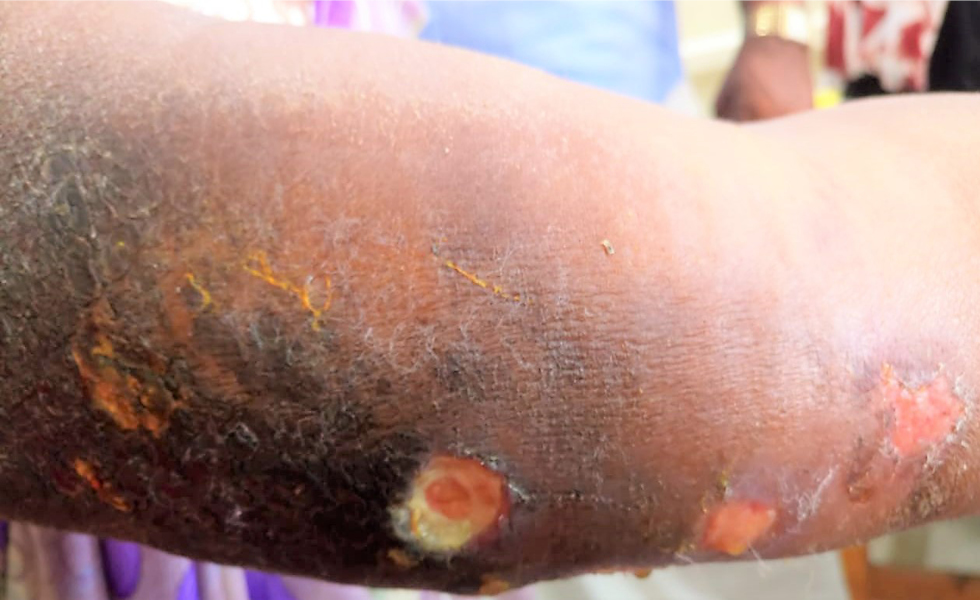
- Ulcerated lobular panniculitis on the left upper arm
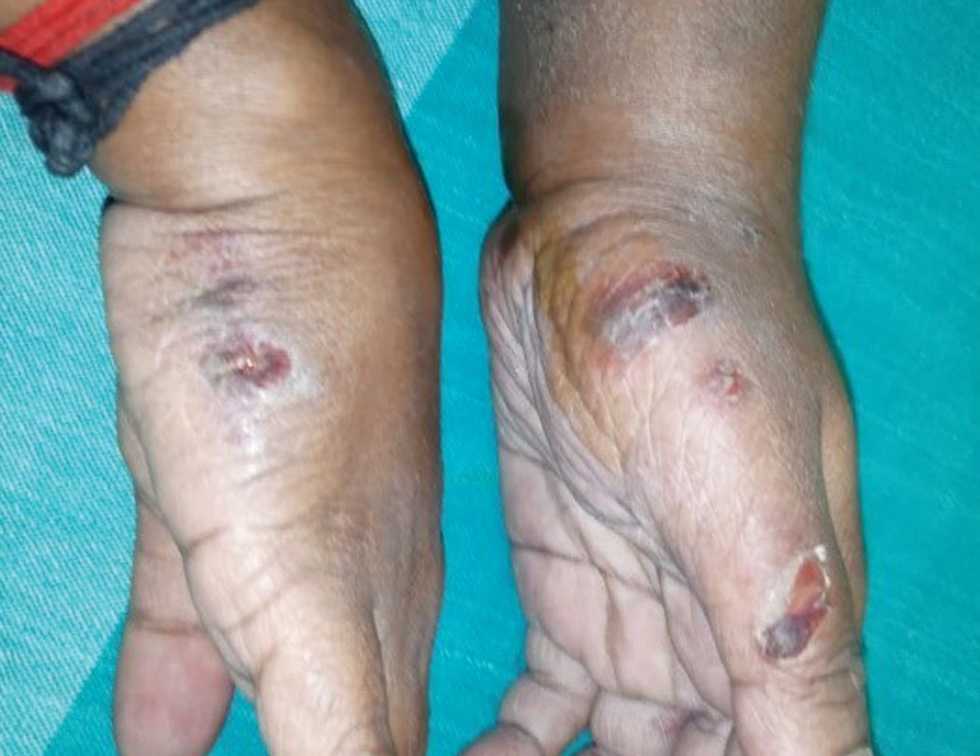
- Multiple ulcers on the radial aspect of the left hand and the ulnar aspect of the right hand
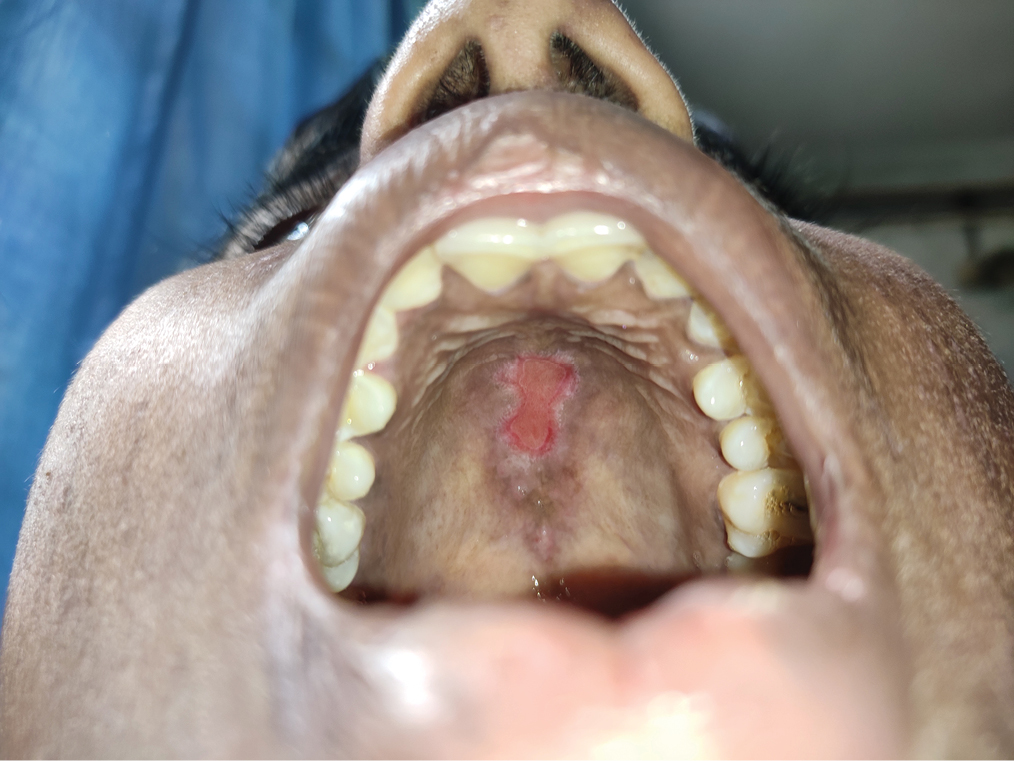
- Erythema and erosion on hard palate
On re-evaluation, the hemogram was normal except for low haemoglobin (9.2 mg/dl). Erythrocyte sedimentation rate and C-reactive protein were normal. Peripheral smear showed no features of haemolysis. Reticulocyte count and serum lactate dehydrogenase levels were within normal limits. Direct Coombs test was negative. Liver function test showed raised aspartate (196 IU/L) and alanine aminotransferases (88 IU/L). Prothrombin time and international normalized ratio were normal. Creatine phosphokinase was elevated to 548 IU/L (24–145 IU/L). Urine routine examination and renal function tests were normal. Repeat serology for human immunodeficiency virus, hepatitis B, and C viruses and anti-nuclear antibody (immunofluorescence) were negative. Anti-double stranded DNA, anti-Smith, anti-histone, anti-U1RNP and anti-PM-Scl antibodies were negative and serum levels of complements (C3 and C4) were normal. Repeat chest X-ray, high-resolution computed tomography thorax and ultrasonogram of abdomen and pelvis were normal. Magnetic resonance imaging of both thighs showed mild oedema and patchy contrast enhancement involving quadriceps femoris muscles, suggestive of myositis. Myositis profile was positive for anti-Mi-2-alpha antibody. A comprehensive evaluation did not reveal any underlying malignancy. Considering the heliotrope rash, symmetrical proximal muscle weakness and elevated muscle enzymes, we made a diagnosis of probable dermatomyositis based on the Bohan and Peter classification.1 Transaminitis was likely due to myositis, as a part of dermatomyositis. Anti-tuberculosis therapy was withdrawn, and she was treated with dexamethasone four mg twice daily intravenously and hydroxychloroquine 200 mg twice daily per orally. As fever persisted and cutaneous ulcers progressed, she was given methyl prednisolone one gm intravenously on three consecutive days. She had a dramatic response. The fever subsided and muscle weakness, cutaneous ulcers and panniculitis resolved completely [Figure 3a to c]. She was continued on daily prednisolone at a dose of 40 mg, tapered by 10 mg per month till it reached 20 mg. Liver enzymes became normal within three weeks. After two months, while on daily 20 mg oral prednisolone and 400 mg hydroxychloroquine, 10 mg methotrexate once weekly was added. Thereafter, prednisolone was tapered by 5 mg per month. While she was on 10 mg prednisolone, she developed a relapse of panniculitis. She presented with multiple painful subcutaneous nodules with ulceration on limbs and trunk [Figure 3d]. The daily dose of prednisolone was immediately increased to 20 mg. As there was no response, she was given two doses of intravenous infusion of 1000 mg rituximab two weeks apart. Lesions responded well to rituximab therapy. She was maintained on prednisolone 10 mg daily and methotrexate 10 mg once weekly. As she developed a minor relapse of skin lesions after one month, the dose of methotrexate was increased to 25 mg weekly. The patient was in remission on last follow up visit.
- Bilateral periorbital post-inflammatory hyperpigmentation
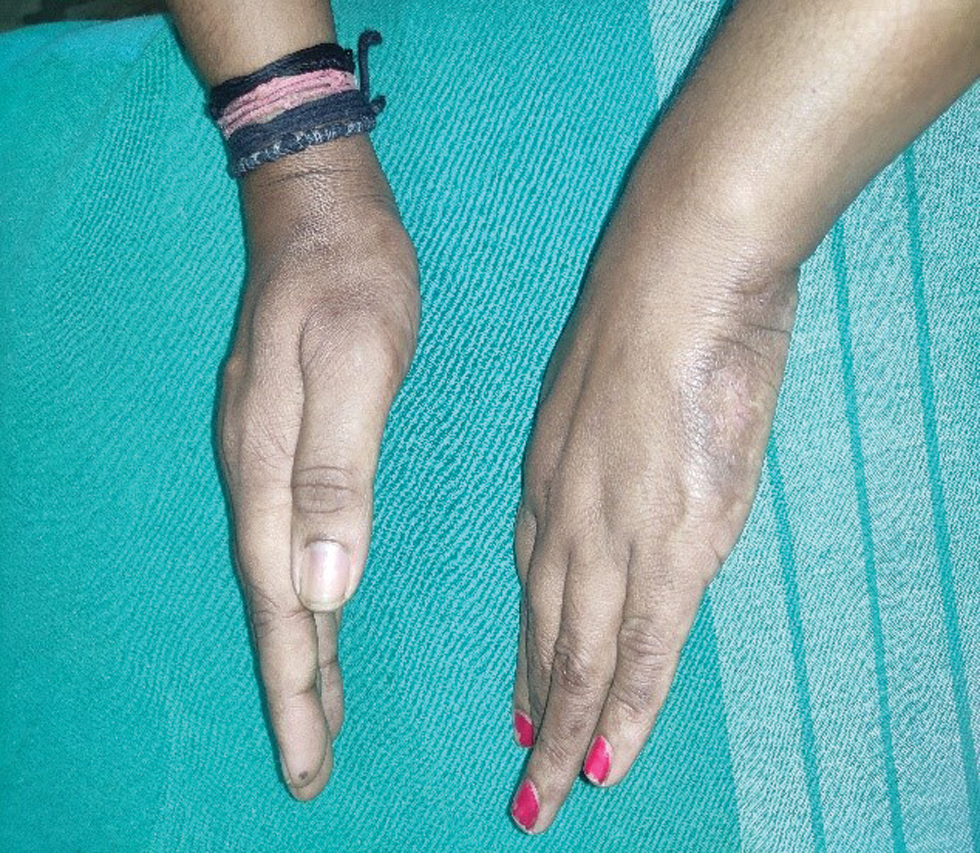
- Post-inflammatory hyperpigmentation following subsidence of cutaneous ulcers on the sides of both hands
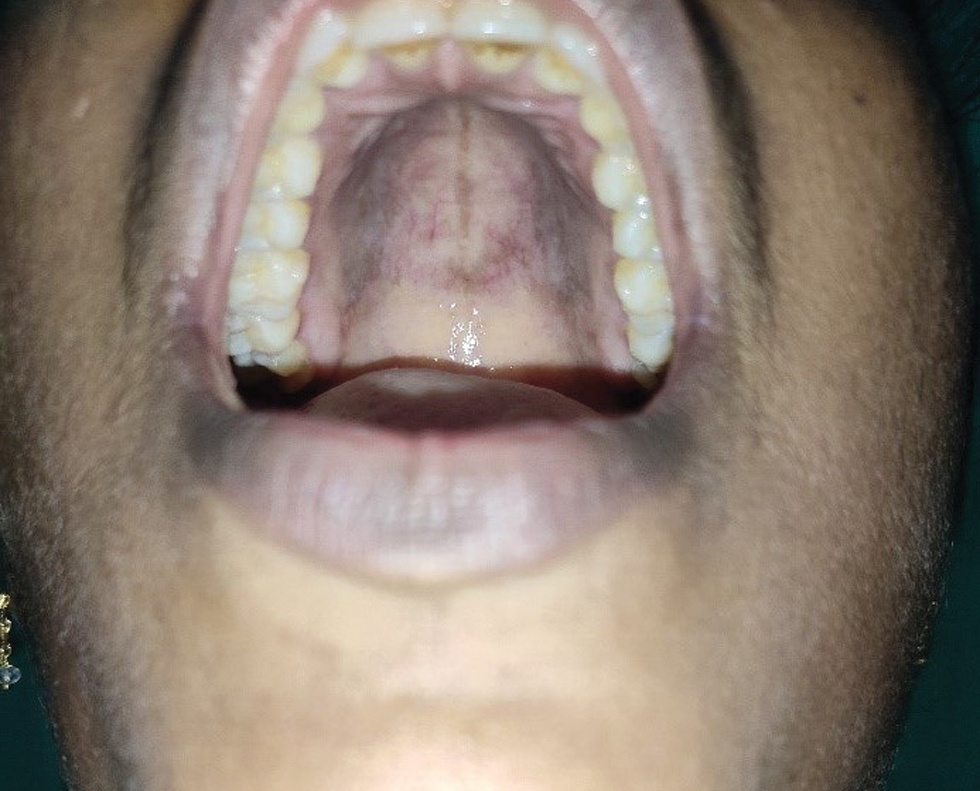
- Post-inflammatory hyperpigmentation on the hard palate
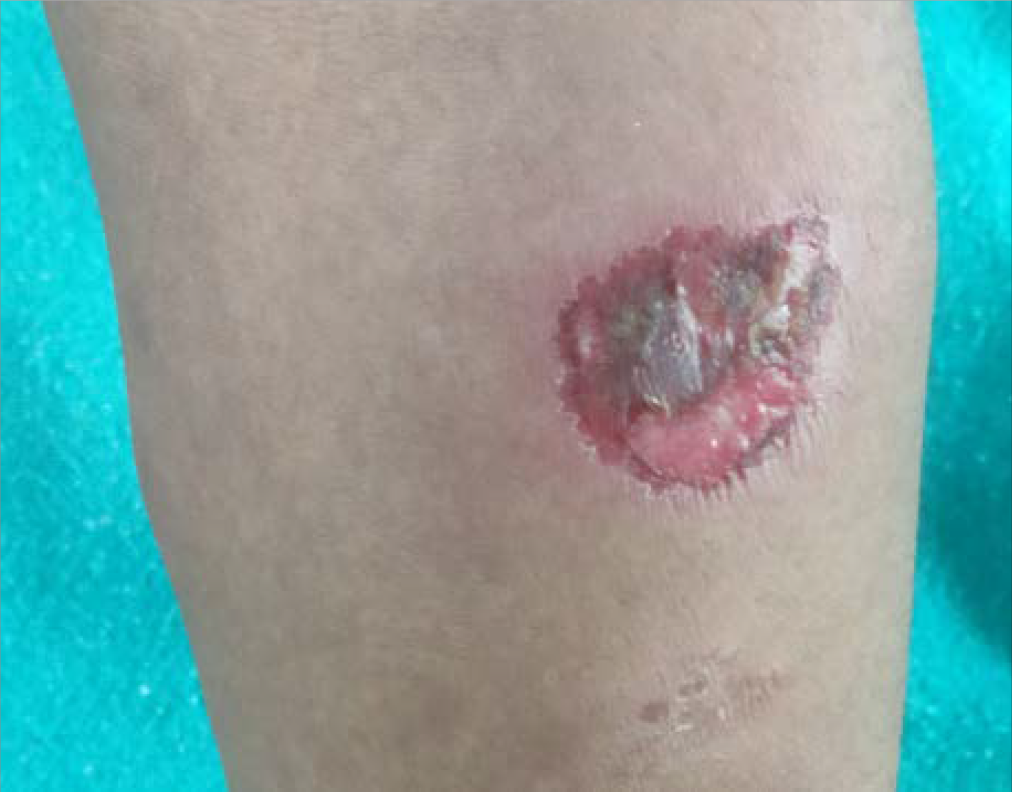
- Subcutaneous nodule with ulceration on the surface of left forearm
The unusual features of our case include ulcerated lobular panniculitis, ulceration of heliotrope rash and cutaneous ulcers in the context of anti-Mi-2-alpha antibody-positive dermatomyositis. Lobular panniculitis, cutaneous ulcers and diffuse subcutaneous oedema though rare can be important diagnostic clues for dermatomyositis.2 Ulcerated heliotrope rash has been recently described in a patient with dermatomyositis associated with anti-melanoma differentiation-associated gene 5(anti-MDA5) antibody.3 A thorough literature search did not reveal any previous reports of ulcerated heliotrope rash in association with anti-Mi-2-alpha antibody-positive dermatomyositis. Painless palatal erosions, fever and weight loss though classically described in systemic lupus erythematosus can be manifestations of dermatomyositis too.4 The asymptomatic palatal erythema with erosion observed in our patient differed from the ovoid palatal patch, described by Bernet et al., in transcription intermediary factor 1-gamma associated dermatomyositis.5 The surface erosion, absence of intermingling white macules, association with anti-Mi-2-alpha antibody and a favourable outcome differentiated the palatal lesion in our patient from the classical ovoid palatal patch described in dermatomyositis.
The exact cause for pleural effusion could not be ascertained in our patient as it was not evident on subsequent evaluation. The magnetic resonance imaging of proximal muscles showed a patchy pattern of muscle inflammation. A patchy or diffuse pattern of muscle inflammation can be seen in dermatomyositis.6 Whether the elevated liver enzymes were a manifestation of dermatomyositis or anti-tuberculosis therapy-induced remains unclear since the therapy itself is well known to produce the same. There are a few reports of anti-tuberculosis therapy being a triggering factor for dermatomyositis.7 We also suspected an exacerbation of clinical manifestations of dermatomyositis with anti-tuberculosis therapy in this case. Our patient had a recurrence of ulcerative panniculitis while on hydroxychloroquine, methotrexate and tapering doses of systemic steroids. The addition of rituximab helped us to achieve prompt resolution. There are very few previous reports on the effectiveness of rituximab in improving the muscle strength and refractory cutaneous lesions of dermatomyositis.8,9
To conclude, lobular panniculitis can be an initial manifestation of dermatomyositis that can precede other features by months or years. Ulcerative lobular panniculitis, ulcerative heliotrope rash and painless palatal erosion are rare manifestations of anti-Mi-2-alpha antibody-positive dermatomyositis. Rituximab could be a promising therapeutic option in dermatomyositis, especially in patients with refractory panniculitis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
References
- Polymyositis and dermatomyositis: Disease spectrum and classification. Indian J Dermatol. 2012;57:366-70.
- [CrossRef] [PubMed] [Google Scholar]
- Covert clues: The non-hallmark cutaneous manifestations of dermatomyositis. Ann Transl Med. 2021;9
- [CrossRef] [PubMed] [Google Scholar]
- Ulcerative heliotrope rash in antimelanoma differentiation-associated gene 5 dermatomyositis. Cutis. 2021;107:E5-E8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentation and evaluation of dermatomyositis. Indian J Dermatol. 2012;57:375-81.
- [CrossRef] [PubMed] [Google Scholar]
- Ovoid palatal patch; a novel finding in dermatomyositis associated with anti-TIF1-γ (p155) antibodies. J Am Acad Dermatol. 2016;152:1049-51.
- [CrossRef] [Google Scholar]
- A comparison of inflammatory myopathies at whole-body turbo STIR MRI. Clin Radiol. 2005;60:261-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology (Oxford). 2017;56:247-54.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab in the treatment of dermatomyositis: An open-label pilot study. Arthritis Rheum. 2005;52:601-7.
- [CrossRef] [PubMed] [Google Scholar]





