Translate this page into:
Ultrasonographic assessment of skin structure according to age
2 Department of Ultrasonography, University of Medicine and Pharmacy "Iuliu Hatieganu," Cluj-Napoca, Romania
3 Department of Pharmacoloy, University of Medicine and Pharmacy "Iuliu Hatieganu," Cluj-Napoca, Romania
4 Department of Histology, University of Medicine and Pharmacy "Iuliu Hatieganu," Cluj-Napoca, Romania
5 Department of Ultrasonography, University of Medicine and Pharmacy �Iuliu Hatieganu,� Cluj-Napoca, Romania
Correspondence Address:
Maria Crisan
Department of Histology/Dermatology, UMPh Iuliu Hatieganu, Cluj-Napoca
Romania
| How to cite this article: Crisan D, Lupsor M, Boca A, Crisan M, Badea R. Ultrasonographic assessment of skin structure according to age. Indian J Dermatol Venereol Leprol 2012;78:519 |
Abstract
Background: High-frequency ultrasound is a noninvasive tool that offers characteristic markers, quantifying the cutaneous changes of the physiological senescence process. Aims: The aim was to assess the changes in skin thickness, dermal density and echogenicity, as part of the ageing process, with different age intervals. Methods : The study was performed on 160 patients, aged 40.4 ± 21.2, divided into four age categories: <20, 21-40, 41-60, 61-80. Ultrasonographic images (Dermascan device) were taken from three sites: dorsal forearm (DF), medial arm (MA), zygomatic area (ZA). We assessed the thickness of epidermis and dermis (mm), number of low, medium, high echogenicity pixels, the ratio between the echogenicity of the upper and lower dermis (LEPs/LEPi), and SLEB (subepidermal low echogenicity band). The statistical analysis was performed using SPSS 15.00. A P value <0.05 was considered significant. Results: On all examined sites, it was found that the dermal thickness increases in the 21 to 40 year interval (P<0.0001). After the 21 to 40 year interval, the number of low echogenic pixels increases significantly, especially on photoexposed sites. High-echogenic pixels follow the same pattern on all examined sites: they increase in the 21 to 40 year interval and decrease in the 3rd and 4th age category. The LEPs/LEPi ratio increases significantly with age, at all sites (P<0.05), due to an increase of hypoechogenic pixels in the upper dermis. Conclusions: High-frequency ultrasound is a noninvasive "histological" tool that can assess the cutaneous structure and age-related changes. It offers imagistic markers, comparable to the histological parameters and also characteristic ultrasonographic markers. Histology remains the gold standard for the investigation of the integumentary system.Introduction
High-frequency ultrasound is a new, noninvasive method that allows an "in vivo assessment0" of the physiological and pathological aspects of the integumentary system. [1] It represents a more desirable and less emotionally involving alternative to skin biopsy, routinely used in the dermatological field.
It also represents an important research tool for the characterization of skin properties with different age intervals, allowing the establishment of an imagistic aging model of the integumentary system. [2] The use of high-frequency ultrasound in dermatology allows a clear identification of the skin layers (epidermis, dermis, subcutis) and thus tissue assessment. At frequencies above 10 MHz, it was proven that the technology provides enough resolution to characterize microstructures. [3],[4],[5]
Cutaneous aging is a complex, biological phenomenon, divided into two components: intrinsic and extrinsic aging. [6] Extrinsic aging is caused by environmental exposure, mainly to UV rays, and is more commonly termed photoaging. On photoexposed sites of the skin, aging involves changes in cellular biosynthetic activity that leads to important disorganization of the dermal matrix. [7] Intrinsic aging, the natural aging process, is genetically determined and represents an inevitable change attributable to the passage of time, characterized by physiologic alterations of the skin structure. In human dermis, intrinsic aging is characterized by three features: dermal atrophy due to collagen loss, degeneration in the elastic fiber network, and loss of hydration. [8]
High-frequency ultrasound allows, as the senescence process progresses, the identification of variations in both skin thickness and echogenicity. The extracellular matrix changes, consisting of variations in dermal density and echogenicity throughout the physiological senescence process, can easily be identified with the use of high-frequency ultrasound. [9]
The purpose of the study is to identify, investigate, and characterize the specific ultrasonographic changes of the cutaneous structure related to different intervals of age and degree of photoexposure.
Methods
Patients
The study was performed during July-October 2011 (Dermatology Clinic, Cluj-Napoca, Romania) on 160 subjects, with a mean age of 40.4 ± 21.2 (STDEV), 50% male and 50% female, divided into four age categories, each category with 40 subjects: <20 , 21-40, 41-60, 61-80 years.
All subjects were submitted to an ultrasonographic evaluation, consisting of the acquirement of ultrasonographic images from three sites: dorsal forearm (DF), medial arm (MA), and zygomatic area (ZA). The dorsal forearm (DF) and zygomatic area (ZA) sites were chosen as prototypes of photoexposed areas, where the dynamics of the extrinsic aging process can be assessed. The ultrasonographic study of the medial arm (MA) site, a photoprotected area, allowed the dynamic study of the intrinsic aging process.
The study was approved by the ethical committee. Every subject was informed about the nature and purpose of the study, and signed an acceptance form before enrolling into the study.
Ultrasonographic evaluation
The ultrasonographic assessment of the integument was performed with a 20 MHz high-frequency Dermascan C device (Cortex Technology, Denmark), that allows the "in vivo" acquirement of cross-sectional images of the skin (B mode) up to 2.5 cm in depth. [10]
The device consists of a transducer, an elaboration system, and a data storing system. The ultrasonic wave is partially reflected at the boundary between adjacent structures and generates echoes of different amplitudes. The intensity of the reflected echoes is evaluated by a microprocessor and visualized as a colored two-dimensional image. [10] The color scale of echogenicity is: white-yellow-red-green-blue-black. On a normal cutaneous image, the epidermal echogenicity appears as a white band, the dermis is expressed as a two color composition: yellow and/or red, and the subcutaneous layer appears either green or black [Figure - 1]a.
 |
| Figure 1b: Subepidermal low echogenicity band (SLEB) Figure 1a: Ultrasonographic aspect of normal skin (Dermascan device, Cortex Technologies) |
The ultrasonographic images are saved and processed with specific image analysis software (Dermavision, Cortex Technology), that has a certain property: the amplitudes of the echoes of the pixels are given as a value on a numerical scale that ranges from 0 to 255. On this scale, the low-echogenity pixel area corresponds to the 0-30 interval, the medium echogenity pixels to 50-150, and the high echogenity pixels to the 200-255 interval. [11]
The gain curve was adjusted to a value of 20 dB, whereas the speed of ultrasound at tissue level was of 1580 m/s. The ultrasonographic gel was applied on the aperture of the ring of the transducer, which was placed perpendicularly to the skin surface for the acquirement of the cross-sectional image. The scanning of the skin was performed on two photoexposed sites of the skin (DF and ZA) and on a photoprotected one (MA).
For every subject, we assessed the thickness of the epidermis and dermis (mm), the number of LEP (low echogenic pixels), MEP (medium echogenic pixels), HEP (high echogenic pixels), SLEB (subepidermal low echogenicity band), LEPs/LEPi ratio (number of low echogenic pixels in the upper dermis/number of low echogenic pixels in the lower dermis). The thickness of the epidermis was obtained by establishing the mean of three measurements performed in A-mode at three different sites of each image (the two extremities of the analyzed image and the center of the image). The thickness of the dermis was obtained in the B-mode, by measuring the distance between the dermo-epidermal and the dermo-hypodermic junction at the same three different sites and by establishing the mean of the three values. By selecting a certain interval from the 0-255 pixel scale, we obtained values corresponding to the low, medium- and high-echogenic pixels, present in the analyzed image. SLEB was defined as a well delimited, subepidermal low echogenicity band (0- 30), situated in the upper dermis, mainly present on photoexposed sites [12] [Figure - 1]b.
Additionally, the LEP were quantified separately in the upper (LEPs) and lower (LEPi) dermis. To separate the two areas, we drew a parallel line to the epidermal entrance echo, dividing the dermis into two equally thick parts. The ratio of the LEP number in the upper and lower dermis (LEPs/LEPi) was calculated.
Statistical analysis
The data we obtained were statistically assessed, based on the ANOVA and Student T test, using the SPSS program version 15.0 (SPSS Inc., Chicago, IL, USA). We evaluated the differences between values referring to different intervals of age at the three examined sites. A P value <0.05 was considered significant.
Results
Epidermis and dermis thickness
The obtained data concerning the epidermis and dermis thickness show different values for the four intervals of age taken into study [Table - 1].
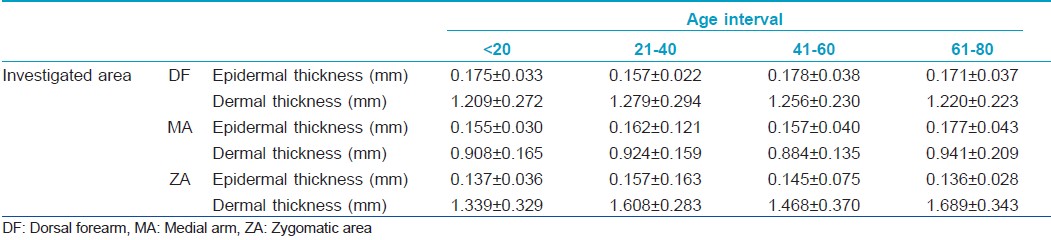
The thickness of the epidermis remains at similar values on all examined sites, except for the epidermis from DF level, where a significant increase is noticed between the 21- to 40- and 41- to 60-year intervals (0.157±0.022 to 1.178±0.038 mm, P=0.035). It can also be noticed that the epidermis tends to decrease after the age of 60 on photoexposed sites (DF, ZA), and to increase on photoprotected ones (MA).
The thickness of the dermis shows certain variations. On all examined sites, the dermal thickness increases in the 21- to 40-year interval. At facial level, from a mean of approximately 1.339±0.329 mm in the <20-year interval, the dermis reaches a value of 1.689±0.343 mm for the subjects taking part in the >60-year interval (P<0.0001). Generally, the dermis increases from the 0- to 20- to the 21- to 40-year intervals and decreases slightly in the 41-60 interval.
Generally, considering the total thickness of the integument, a significant increase may be noticed especially at facial site (P<0.0001). Also, comparing the two extreme age intervals, 0-20 and 61-80, we can notice that the integument is thinner in the 61- to 80-year interval at the level of dorsal forearm, has comparable values on the medial arm and increases at facial level.
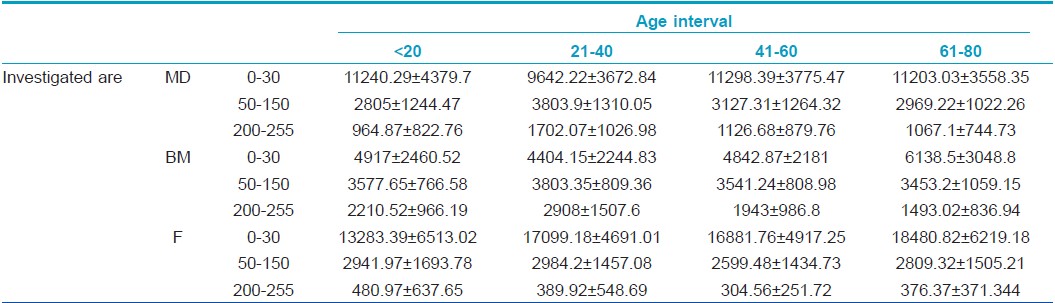
Assessment of low, medium, and high echogenicity pixels
The number of hypoechogenic pixels shows a significant variation in the case of all three examined sites: hypoechogenic pixels decrease significantly on the dorsal forearm in the 21- to 40-year interval compared to the 0-20 interval (9642.22±3672 vs. 11240.29±4379, P<0.05) and increase significantly in the 61-80 interval. At medial arm level, hypoechogenic pixels increase significantly from the 21- to 40-year age interval to the 61-80 one (4404.15±2244 vs. 6138.5±3048, P=0.013) whereas at facial level, there is a significant increase of low echogenic pixels from the first to the fourth age category (13283.39±6513 vs. 18480.82±6219, P<0.0001).
The number of medium echogenic pixels varies in a statistically significant manner at dorsal forearm level: they increase in the 21- to 40-year interval (P=0.02) and decrease in subjects belonging to the 41-60 and 61-80 interval (P=0.01). At facial and medial arm level, medium echogenic pixels also tend to increase in the second age category, followed by a decrease in the third age interval. At medial arm level, in all age categories, the amount of medium echogenic pixels has higher values than on the other two-photoexposed sites: facial level and dorsal forearm.
The hyperechogenic pixels increase significantly at the dorsal forearm level in the 21- to 40-year interval (964.87±822 vs. 1702.075±1026, P=0.01) followed by a decrease in the 41-60 interval (P=0.03) and the last age category, >60. At facial site, high echogenic pixels decrease in the 21-40 interval (389.92±548 vs. 480.97±637, P<0.05) and 41-60 interval of age and increase after the age of 60 (376.37 ±371 P<0.05). At medial arm level, there is a significant increase of high echogenic pixels in the 21- to 40-year interval (2908.67±1507 vs. 2210.52±966, P=0.03) followed by a decrease of HEP from the 21- to 40-year age interval to the 61-80 one (2908.67±1507 vs. 1493.025±836, P=0.026) [Table - 2].
Assessment of the LEPs/LEPi ratio
The ultrasound study shows different echogenicity degrees for the upper (LEPs) and lower (LEPi) dermis with a consequent variation of the LEPs/LEPi ratio [Table - 3].
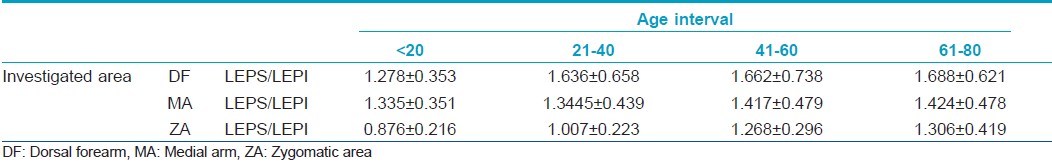
The LEPs/LEPi ratio displays a significant growth with age, at all three examined sites (P<0.05), due to a significant growth of hypoechogenic pixels in the upper dermis [Figure - 2].
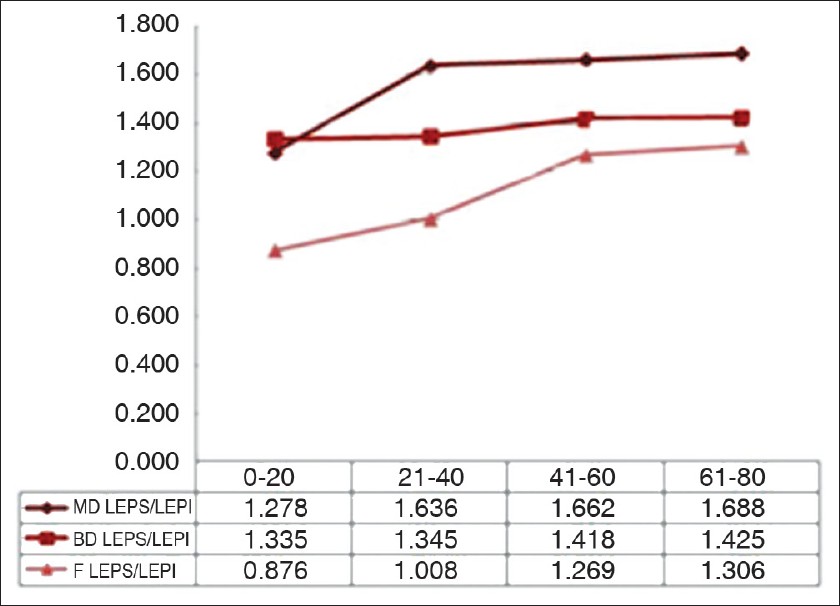 |
| Figure 2: Variation of the LEPs/LEPi ratio on the three examined sites in the four age categories |
Assessment of subepidermal low echogenicity band
SLEB was qualitatively assessed by identifying the presence of the low echogenic band in the acquired images. We noticed the presence of SLEB in all subjects over the age of 20, especially on photoexposed sites (DF, ZA).
Discussion
Ultrasonography is well-established clinical medicine as a noninvasive method of diagnosis. During the past decade, diagnostic ultrasonography has been extended to clinical dermatology as well. Classical ultrasound (7.5-10 MHz) is not common in dermatology, due to a lack of detail, but ultrasound systems with probes of at least 20 MHz can provide useful information regarding tumoral extension, inflammatory infiltrate, etc. High-frequency ultrasound in dermatology is also an accurate method for determining skin thickness and collagen content in various anatomical regions, with different intervals of age. [1],[13],[14] Skin thickness is considered an objective, physiological parameter that allows the assessment of the influence of endogenous or environmental factors, such as UV-rays at tissue level. [10] Many techniques were used for assessing skin thickness: pulsed ultrasound, conventional ultrasound, skin-fold measurements etc. [15],[16] High-frequency ultrasound allows a highly accurate, objective, noninvasive assessment of the epidermal and dermal thickness of the integument, regardless of the site of examination. Even though there are numerous studies regarding the imagistic assessment of the cutaneous aging process, this research has an original approach, is performed on a greater number of subjects (160), quantifies concomitantly several markers and offers results that are in concordance with the field literature. From the histological point of view, chronologically aging skin is characterized by little changes in epidermal thickness and integrity of the stratum corneum. [17] The study has shown that the epidermal thickness, assessed by high-frequency ultrasound, does not vary in a significant manner on any of the examined sites, having values between 0.15 and 0.17 mm. Histological data points out a dermal loss of volume, especially on photoprotected sites, induced by various intrinsic and extrinsic factors. [18]
It was also noticed that the dermal thickness has a tendency to increase in the 21- to 40-year interval on all examined sites and decreases after the age of 40. This particular tendency indicates the presence of intense synthesis processes until the age of 40, followed by the degenerative phenomena of the extracellular matrix. A significant growth of the dermis with age is noticed at facial level, a highly photoexposed area [19],[20],[21] [Figure - 3].
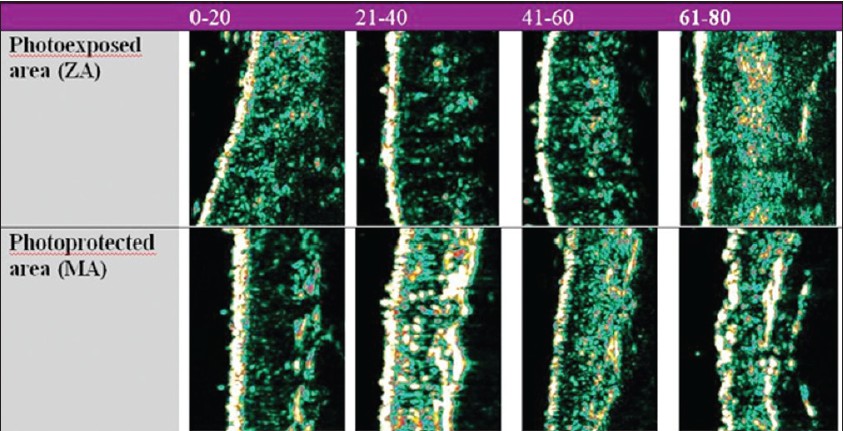 |
| Figure 3: Age-related ultrasonographic aspects of the skin proving objective changes of skin thickness and dermal density |
Studies have revealed that in highly photoexposed skin, the amount of glycosaminoglycans and proteoglycans is increased in the dermal ground substance, while collagen fibers decrease. The thinning of the skin with aging results as a consequence of a decrease in dermal collagen synthesis. [22],[23]
Also, we noticed that the dermis is thinner on photoprotected sites than on the photoexposed ones in all age intervals. The data we obtained was correlated with literature in the field. [24]
The echogenicity of the skin is given by the extracellular matrix density. Skin echogenicity varies significantly with age, correlated with local degradation processes that occur as the senescence process evolves. In time, the dermis becomes hypocellular, the number of fibroblasts decreases, and a dermal volume loss is noticed. [25],[26],[27],[28] Also, collagen fibers undergo a degeneration process, elastic fibers become disorganized and most of the subepidermal fibers are resorbed. [29]
Concerning the assessment of pixels, after the 21- to 40-year interval, we notice a significant increase of the number of low echogenic pixels (LEP), especially on photoexposed sites such as dorsal forearm and zygomatic area that is due to the accumulation of elastotic material, increase of glycosaminoglycans and proteoglycans amount, aspects visible on histological sections. LEP, according to literature, quantify the degree of cutaneous hydration, inflammatory processes, solar elastosis, collagen degeneration [2] [Figure - 4].
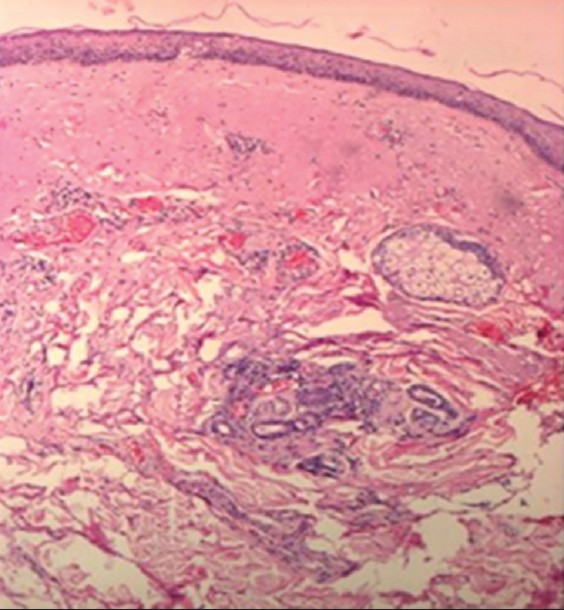 |
| Figure 4: Histological section: skin elastosis (H and E, ×10) |
We also mention that SLEB becomes visible mainly after the 21- to 40-year interval especially on photoexposed sites. SLEB is the correspondent of both the histologically identified Grenz zone (collagen neofibrills and glycosaminoglycans) and elastosis, which are present on photoexposed sites. Grenz zone, a very thin band located below the epidermis is an area of tissue regeneration of the actinic-induced lesions. According to literature, SLEB may be considered an objective marker of the photoaging process. [30],[31] Medium echogenic pixels (MEP) have higher values at medial arm level compared to the photoexposed sites. Together with the high echogenic pixels (HEP), they quantify the proteic structures as well as the degree of assembly into microfibrills or mature fibers. [32]
High echogenic pixels (HEP) follow the same pattern on all three examined sites: they increase in the 21- to 40-year interval and decrease gradually in the third and fourth age category. The decrease is significant in the 41-60 interval in all examined sites. Also, it was noticed that on photoprotected sites, the mean value of the HEP is significantly higher than on photoexposed ones; therefore, HEP can be considered an imagistic marker of the intrinsic aging process.
The LEPs/LEPi ratio increases in all age categories, on all examined sites, having the most significant increase at facial and dorsal forearm level, the highest photoexposed sites. In these two sites, we noticed a significant increase of the LEPs in the upper dermis, and a decrease of the LEPi. The LEPs/LEPi ratio allows an assessment of the density and integrity of the extracellular matrix, both from the upper and lower dermis, which vary according to age, UV-ray exposure, and therapy. [32]
According to literature, LEPs/LEPi is considered an objective, imagistic marker for the photoaging process. The dynamics of the repartition of the pixels amplitude in the case of the 20-40 "critical interval" on all studied sites indicates the initiation of important morphological, structural, and functional reactions at cutaneous level. [32] We consider that the photoinduced cutaneous pathology that may usually be evidenced after the age of 45, as well as the local, cutaneous changes, can be improved through efficient protection measures applied until the age of 40.
The identification of a general, ultrasonographic pattern regarding both intrinsic and extrinsic aging, may be of great use for the noninvasive assessment of the regenerative effect of various personalized cutaneous antiaging therapies. [33] High-frequency ultrasound can therefore be used for preventive monitoring of skin, prohpylaxis of the skin aging process, and personalized therapy assessment. Also the variation of the identified ultrasonographic parameters can be used for the noninvasive evaluation of the efficacy of different topical therapies in chronic inflammatory disorders (morphea, scleroderma, psoriasis). The histological changes suggesting an improvement or deterioration after therapy, assessed noninvasively by high-frequency ultrasound, occur prior to the clinical aspects; therefore we can assess the efficacy of a topical therapy before the clinical improvements appear.
Conclusions
High-frequency ultrasound is a noninvasive "histological" tool that can assess various cutaneous parameters such as skin thickness, dermal density, and age-related echogenicity, but histology remains the gold standard for the study of the integumentary system. The identification of the dynamics of the ultrasonographic parameters with age can be used for future evaluation of the efficacy of various personalized topical anti-aging therapies.
Acknowledgments
The authors gratefully acknowledge Cortex Technologies, Denmark, for allowing the use of the 20 MHz high-frequency DERMASCAN device in the elaboration of the study. This study was performed as part of a financed contract of the University of Medicine and Pharmacy "Iuliu Hatieganu", Cluj-Napoca, Romania, thru the internal grant nr. 22714/7/06.10.2011
| 1. |
Serup J, Keiding J, Fullerton A, Gniadecka M, Gniadecki R. High-frequency ultrasound examination of skin: Introduction and guide. In: Serup J, Jemec GB, editors. In vivo examination of the skin: Handbook of non-invasive methods. Boca Raton, FL: CRC Press; 1995. p. 239-56.
[Google Scholar]
|
| 2. |
Gniadecka M, Jemec GB. Quantitative evaluation of chronological aging and photoaging in vivo: Studies on skin echogenicity and thickness. Br J Dermatol 1998;139:815-21.
[Google Scholar]
|
| 3. |
Cammarota T, Pinto F, Magliaro A, Sarno A. Current uses of diagnostic high-frequency US in dermatology. Eur J Radiol 1998;27 Suppl 2:S215-23.
[Google Scholar]
|
| 4. |
Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol 2002;12:390-9.
[Google Scholar]
|
| 5. |
Sandby-Møller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: Relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol 2003;83:410-3.
[Google Scholar]
|
| 6. |
Itahana K, Campisi J, Dimri GP. Mechanisms of cellular senescence in human and mouse cells. Biogerontology 2004;5:1-10.
[Google Scholar]
|
| 7. |
Puizina-Iviæ N. Skin aging. Acta Dermatovenerol Alp Panonica Adriat 2008;17:47-54.
[Google Scholar]
|
| 8. |
Uitto J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J Drugs Dermatol 2008;7:s12-6.
[Google Scholar]
|
| 9. |
Gniadecka M. Effects of ageing on dermal echogenicity. Skin Res Technol 2001;7:204-7.
[Google Scholar]
|
| 10. |
Seidenari S, Pagnoni A, Di Nardo A, Giannetti A. Echographic evaluation with image analysis of normal skin: Variations according to age and sex. Skin Pharmacol 1994;7:201-9.
[Google Scholar]
|
| 11. |
Gniadecka M, Serup J, Søndergaard J. Age-related diurnal changes of dermal oedema: Evaluation by high-frequency ultrasound. Br J Dermatol 1994;131:849-55.
[Google Scholar]
|
| 12. |
Gniadecka M, Gniadecki R, Serup J, Søndergaard J. Ultrasound structure and digital image analysis of the subepidermal low echogenic band in aged human skin: Diurnal changes and interindividual variability. J Invest Dermatol 1994;102:362-5.
[Google Scholar]
|
| 13. |
Schmid-Wendtner MH, Burgdorf W. Ultrasound scanning in dermatology. Arch Dermatol 2005;141:217-24.
[Google Scholar]
|
| 14. |
Stojanoviæ S, Poljacki M, Ros T. Diagnostic importance of ultrasound in dermatology. Med Pregl 2002;55:392-6.
[Google Scholar]
|
| 15. |
Tan CY, Statham B, Marks R, Payne PA. Skin thickness measurement by pulsed ultrasound: Its reproducibility, validation and variability. Br J Dermatol 1982;106:657-67.
[Google Scholar]
|
| 16. |
Laurent A, Mistretta F, Bottigioli D, Dahel K, Goujon C, Nicolas JF, et al. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine 2007;25:6423-30.
[Google Scholar]
|
| 17. |
Lavker RM, Zheng PS, Dong G. Aged skin: A study by light, transmission electron, and scanning electron microscopy. J Invest Dermatol 1987;88:44-51.
[Google Scholar]
|
| 18. |
Montagna W, Carlisle K. Structural changes in ageing skin. Br J Dermatol 1990;122:61-70.
[Google Scholar]
|
| 19. |
Ha RY, Nojima K, Adams WP Jr, Brown SA. Analysis of facial skin thickness: Defining the relative thickness index. Plast Reconstr Surg 2005;115:1769-73.
[Google Scholar]
|
| 20. |
Takema Y, Yorimoto Y, Kawai M, Imokawa G. Age-related changes in the elastic properties and thickness of human facial skin. Br J Dermatol 1994;131:641-8.
[Google Scholar]
|
| 21. |
Dumas M, Langle S, Noblesse E, Bonnet-Duquennoy M, Pelle de Queral D, Tadokoro T, et al. Histological variation of Japanese skin with aging. Int J Cosmet Sci 2005;27:47-50.
[Google Scholar]
|
| 22. |
Affinito P, Palomba S, Sorrentino C, Di Carlo C, Bifulco G, Arienzo MP, et al. Effects of postmenopausal hypoestrogenism on skin collagen. Maturitas 1999;33:239-47.
[Google Scholar]
|
| 23. |
Brincat M, Moniz CJ, Studd JW, Darby A, Magos A, Emburey G, et al. Long-term effects of the menopause and sex hormones on skin thickness. Br J Obstet Gynaecol 1985;92:256-9.
[Google Scholar]
|
| 24. |
Pellacani G, Seidenari S. Variations in facial skin thickness and Echogenicity with site and age. Acta Derm Venerol 1999;79:366- 9.
[Google Scholar]
|
| 25. |
Beauregard S, Gilchrest BA. A survey of skin problems and skin care regimes in the elderly. Arch Dermatol 1987;123:1638-43.
[Google Scholar]
|
| 26. |
Lapière CM. The ageing dermis: The main cause for the appearance of 'old' skin. Br J Dermatol 1990;122:5-11.
[Google Scholar]
|
| 27. |
Mine S, Fortunel NO, Pageon H, Asselineau D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: A new view of skin morphogenesis and aging. PLoS One 2008;3:e4066.
[Google Scholar]
|
| 28. |
Kurban RS, Bhawan J. Histologic changes in skin associated with aging. J Dermatol Surg Oncol 1990;16:908-14.
[Google Scholar]
|
| 29. |
Yaar M, Gilchrest BA. Aging of skin. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick's Dermatology in General Medicine. vol. 2. New York: McGraw-Hill; 2003. p. 1386-98.
[Google Scholar]
|
| 30. |
Fligiel SE, Varani J, Datta SC, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol 2003;120:842-8.
[Google Scholar]
|
| 31. |
Sandby-Møller J,Wulf HC. Ultrasonographic subepidermal low-echogenic band, dependence of age and body site. Skin Res Technol 2004;10:57-63.
[Google Scholar]
|
| 32. |
Crisan M, Cattani C, Badea R, Mitrea P, Florea M, Crisan D, et al. Modelling cutaneous senescence process. Lect Notes Comput Sci 2010;6017:215-24.
[Google Scholar]
|
| 33. |
Kruglikov IL, Sontag W. Ultrasound of 10 MHz frequency as a novel strategy for skin anti-aging therapy. Med Hypotheses 2010;74:620-1.
[Google Scholar]
|
Fulltext Views
6,508
PDF downloads
3,569





