Translate this page into:
Ultrasound assessment of enthesis thickness in psoriasis and psoriatic arthritis: A cross-sectional study
2 Department of “Medicina dei Sistemi”, Rheumatology, Allergology and Clinical Immunology, University of Rome Tor Vergata, Viale Oxford, 81, 00100 Rome, Italy
Correspondence Address:
Pier Luigi Saraceni
Department of Clinical Dermatology, San Gallicano Dermatological Institute, Via Elio Chianesi 53, 00144, Rome
Italy
| How to cite this article: Graceffa D, Bonifati C, Lora V, Saraceni PL, De Felice C, Chimenti MS, Perricone R, Morrone A. Ultrasound assessment of enthesis thickness in psoriasis and psoriatic arthritis: A cross-sectional study. Indian J Dermatol Venereol Leprol 2019;85:175-181 |
Abstract
Background: The inflammatory involvement of the enthesis in the course of psoriasis is accompanied by structural abnormalities detectable by ultrasound. The most common of these abnormalities is the thickening of the tendon at the insertion site.
Aims: The aim of the present study was to compare the thickness of entheses of patients with psoriatic arthritis, only skin psoriasis, and healthy controls.
Methods: A cross-sectional study was conducted in a cohort of patients affected with either only skin psoriasis or psoriatic arthritis as well as in a control group. Eight entheses sites were scanned by ultrasound bilaterally. The following entheseal characteristics were collected and recorded in a predefined database: entheseal thickness, bone erosions, enthesis calcifications (enthesophytes), presence of blood flow, and presence of bursitis. All the detected entheseal changes were scored, and the data was statistically analyzed.
Results: The major differences in enthesis thickness between only skin psoriasis and psoriatic arthritis patients were found at the following sites: (i) olecranon tuberosity, (ii) superior pole of the patella, and (iii) medial epicondyle of femur. The thickness of the medial collateral ligament at the site of the femoral origin was increased in psoriatic arthritis, but not in both only skin psoriasis and healthy controls. The score obtained by adding the thickness of all the 8 examined entheses for each patient showed significant differences among the three groups (psoriatic arthritis: 81.3; only skin psoriasis 74.4; Controls: 67.6; P < 0.0001). Interestingly, we found that in psoriatic arthritis patients, the highest enthesis thickening was seen in entheses affected by bone erosions.
Limitations: The small sample of patients studied is a limiting factor in this study.
Conclusions: Our data demonstrated that the ultrasound measurement of the enthesis thickness enables a distinction between patients with psoriatic arthritis from those with only skin psoriasis. It is a useful method to improve diagnostic accuracy, especially in patients without clear clinical signs of enthesitis.
Introduction
Enthesitis is an inflammation at the insertion of ligaments, tendons, or joint capsules to bone. It is a typical feature of spondyloarthritis (SpA), a family of inflammatory rheumatic diseases including psoriatic arthritis that share common clinical features as dactylitis, peripheral arthritis, and involvement of spine joints.[1],[2]
Enthesitis is the earliest event in the course of psoriatic arthritis and could be the only clinical manifestation of the disease.[1] Enthesitis has been hypothesized to be the result of an exaggerated innate immunity response that plays a pivotal role in directing the subsequent adaptive immune response.[3] The clinical assessment of enthesitis is predominantly performed by eliciting tenderness at the entheses' sites. Specific clinical tools have been validated to assess enthesitis such as the Leeds Enthesitis Index (LEI) and the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES).[4],[5]
However, an entheseal involvement is often misdiagnosed and undertreated in many psoriasis patients.[6]
Musculoskeletal ultrasound in combination with power Doppler is a valid and reliable technique for the diagnosis and follow-up of patients with psoriatic arthritis with enthesis involvement.[7],[8],[9]
In the past decades, different ultrasound enthesitis scores have been developed. The most used are: (i) Glasgow Ultrasound Enthesitis Scoring System (GUESS) which examines posterior and inferior pole of the calcaneus, superior and inferior pole of patella; and (ii) Madrid Sonographic Enthesis Index (MASEI) which assesses posterior and inferior pole of the calcaneus, superior and inferior pole of patella, tibial tuberosity and olecranon tuberosity.[7],[9],[10]
Methodological complexity and inter-observer variability are the main factors that limit the use of the above cited scores in clinical practice. Furthermore there is no clarity on the meaning to be attributed to each elemental lesion, and which of these best discriminate between inflammatory and degenerative processes affecting the osteotendinous junction.[7],[9],[11],[12],[13]
In our experience, the ultrasound measurement of the enthesis thickness represents the best tool for the assessment of enthesitis for several reasons: (i) the thickening may be considered an early sign of inflammation compared to the presence of hypervascularization, calcifications, and erosions; (ii) it is an operator-independent method; and (iii) finally it is reliable and easy to use.[14],[15]
Aim of the study
The aim of the present study was to compare the ultrasound measurement of the thickness of 8 enthesis sites between patients with psoriatic arthritis, only skin psoriasis, and healthy controls, to verify if it is an effective and reliable tool for improving diagnostic accuracy, especially in patients without clear clinical signs of enthesitis. Our ultrasound assessment included the evaluation of two sites never considered so far: (i) the lateral epicondyle of the elbow and (ii) the medial epicondyle of femur.
Methods
This was a cross-sectional study conducted in a cohort of patients attending our outpatient dermatology clinic for psoriasis in Rome.
The study was approved by the local ethics committee and was conducted in accordance with the ethical principles of the Declaration of Helsinki.
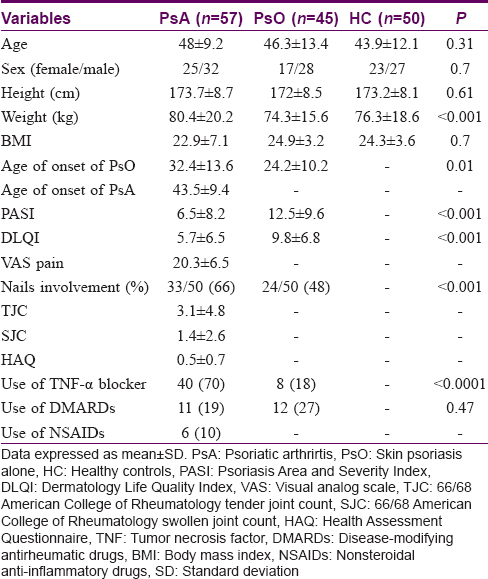
Demographical, clinical, and treatment-related characteristics of the study population are shown in [Table - 1].
Forty-five consecutive patients with only skin psoriasis and 57 with psoriatic arthritis (without clinical signs of active enthesitis) were examined between December 2014 and December 2015. The diagnosis of inflammatory arthritis was made by the consultant rheumatologists (GD and CMS) based on detailed history and physical examination. Patients were classified as affected by psoriatic arthritis in agreement with CASPAR criteria.[16] The diagnosis of skin psoriasis was confirmed by a trained dermatologist (BC).
All patients with psoriatic arthritis were under systemic therapy at the time of ultrasound examination – 40 with biologic DMARDs, 11 with conventional DMARDs, and 6 with NSAIDs.
Twenty of 45 only skin psoriasis patients were under systemic treatment, and the others were treated only with topical medications or UVB.
Patients with clinically active enthesitis (defined as tenderness and/or swelling at the site of an enthesis) were excluded to better explore subclinical entheseal involvement.
Fifty age and sex-matched individuals selected from the hospital staff served as healthy controls. All participants gave their signed informed consent before inclusion in the study.
All ultrasound examinations and entheses thickness measurements were performed by a rheumatologist, blinded to clinical diagnosis, (DG) using a MyLab70 (EsaoteSpA, Genoa, Italy) equipped with a 6–18 MHz broadband linear transducer.
The following 8 enthesis sites were scanned bilaterally: lateral epicondyle, olecranon tuberosity, superior pole of the patella, inferior pole of the patella, tibial tuberosity, medial epicondyle of femur (origin of the medial collateral ligament), superior pole of the calcaneus, and inferior pole of the calcaneus. The sonographer examined morphological and structural abnormalities in B mode, as well as the vascularization with power doppler at bony insertions in both longitudinal and transverse planes; the scan images were stored.
The following entheseal characteristics were collected and recorded in a predefined database: entheseal thickness, bone erosions, enthesis calcifications (enthesophytes), presence of blood flow, and presence of bursitis.
Entheseal thickness was measured at the point of maximal thickness, 2 mm proximal to the bone insertion, and the sum of the thickness of the 8 entheses of each patient was calculated and stored (thickness total score).
The presence of enthesophytes was assessed using a semi-quantitative score from 1 to 3 for each enthesis according to previous studies and the total obtained for each patient yielded a calcification score.[6],[9]
Bone erosion was defined as an interruption of the cortical bone assessed in both longitudinal and transverse planes.
The normal ultrasound features and thickness of the entheses examined have been previously described.[7],[9],[17],[18]
Power Doppler settings were standardized with a Doppler frequency of 8 MHz and pulse repetition frequency of 0.5 kHz.
The sample was not sized for a powerful statistical analysis so, results should be interpreted cautiously. Continuous variables were described as median (range) or mean ± SD according to the distribution. Comparisons among different groups were carried out using Kruskal Wallis analysis (KW), the Mann–Whitney U-test and Pearson's Chi-square as necessary. Pearson coefficient was used for correlations. In all statistical analyses, significance was defined as P < 0.05. Statistical analyses were performed using the GraphPad Prism 5.02 version (GraphPad software Inc, La Jolla, CA, USA).
Results
Differences in enthesis thickness across the groups
The respective differences in enthesis thickness have been summarized in [Table - 2].
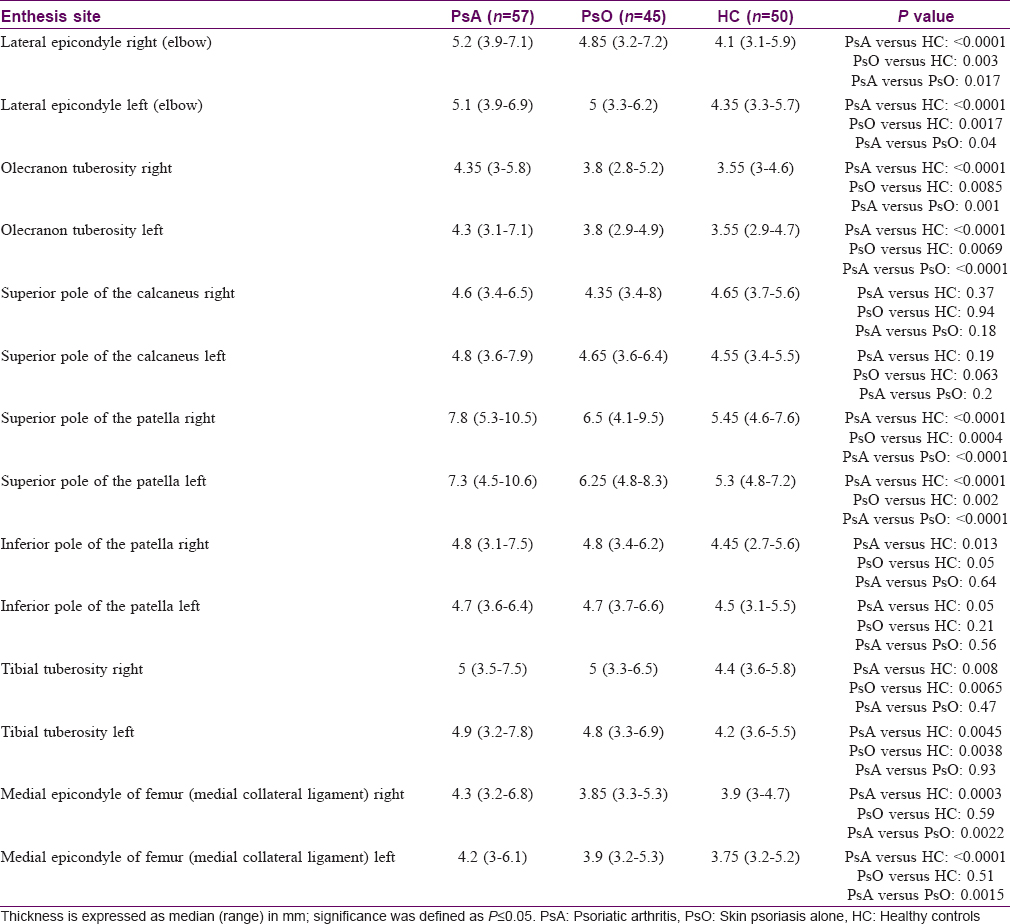
- Elbow: Ultrasound examination of the lateral epicondyle showed a significant difference in the enthesis thickness bilaterally across the three groups (KW: P < 0.0001). In particular, we found the highest difference comparing the right lateral epicondyle of psoriatic arthritis patients with the healthy controls group [psoriatic arthritis: 5.2 mm (3.9–7.1); healthy controls: 4.1 mm (3.1-5.9); P < 0.0001]. A difference of approximately 0.7 mm at the lateral epicondyle bilaterally was found between only skin psoriasis group and healthy controls (P = 0.003). Only slight differences emerged comparing only skin psoriasis and psoriatic arthritis. The olecranon tuberosity enthesis thickness was bilaterally higher in psoriatic arthritis group compared to only skin psoriasis and healthy controls [psoriatic arthritis: 4.3 mm (3–5,8); only skin psoriasis: 3.8 mm (2.8–5.2); healthy controls: 3.5 mm (3–4.6). The following significances were found when comparisons among the different groups were performed: Psoriatic arthritis vs healthy controls P < 0.0001; psoriatic arthritis vs only skin psoriasis P = 0.001, only skin psoriasis vs healthy controls P = 0.0085
- Knee: At the four knee entheses examined, we found relevant differences across the three groups. The highest thickness difference was between the right superior pole of the patella of psoriatic arthritis patients and healthy controls [psoriatic arthritis: 7.8 mm (5.3–10.5); healthy controls: 5.4 mm (4.6–7.6); P < 0.0001]. psoriatic arthritis group differed from only skin psoriasis at approximately 1.3 mm bilaterally (P < 0.0001). At the medial epicondyle of femur, there was bilaterally a significant difference between psoriatic arthritis and healthy controls and between psoriatic arthritis and only skin psoriasis, respectively, but not between only skin psoriasis and healthy controls [significance: Psoriatic arthritis vs healthy controls, P = 0.0003; psoriatic arthritis vs only skin psoriasis, P = 0.0022; only skin psoriasis vs healthy controls, P = 0.59]. The thickness differences detected at the origin and insertion of the patellar ligament emerged mainly by comparing the tibial tuberosity insertion of psoriatic arthritis and healthy controls [psoriatic arthritis: 5 mm (3.5–7.5); healthy controls: 4.4 (3.6–5.8); P = 0.008]
- Heel: the analysis of the measurements carried out at the superior pole of the calcaneus showed only slight statistically not significant differences among the three groups (KW: P =0.4). At the inferior pole of the calcaneus, we found a slight but statistically significant difference among the three groups (KW: P < 0.0001), with the most relevant difference between patients with psoriatic arthritis and controls (0, 8 mm; P < 0.0001).
Thickness total score
The comparative analysis of the score obtained by summing the thickness of the 8 entheses for each patient showed statistically significant difference among the three groups with a median of 81.3 mm (66.7–98.5) for psoriatic arthritis patients and respectively of 74.4 mm (63.6–87.6), and 67.6 mm (60.6–80.7) for only skin psoriasis group and controls (KW: P < 0.0001).
The thickness total score correlates with the number of erosions in psoriatic arthritis group but not in the only skin psoriasis patients and with the calcification score in psoriatic arthritis, only skin psoriasis and healthy controls [Erosions: Psoriatic arthritis Spearman r = 0.44, P = 0.0006; only skin psoriasis Spearman r = 0.28, P = 0.07 (n.s.); Calcification score: Psoriatic arthritis Spearman r = 0.6, P < 0.0001; only skin psoriasis Spearman r = 0.36, P = 0.02; healthy controls Spearman r = 0.38, P = 0.01].
Interestingly, the thickness total score correlates with the BMI of the only skin psoriasis patients and controls [only skin psoriasis Spearman r = 0.42, P = 0.006; healthy controls Spearman r = 0.33, P = 0.02] but not with the BMI of the psoriatic arthritis group [Spearman r = 0.17, P = 0.18 (n.s.)]
A slight correlation was found between the thickness total score and the age of patients with only skin psoriasis and psoriatic arthritis [psoriatic arthritis Spearman r = 0.29, P = 0.03; only skin psoriasis Spearman r = 0.33, P = 0,03]. No correlation was found between the thickness total score and the age of onset of only skin psoriasis and psoriatic arthritis.
Finally, the thickness total score strictly correlates with the MASEI index in the three groups [psoriatic arthritis Spearman r = 0.63, P < 0.0001; only skin psoriasis Spearman r = 0.52, P = 0.0005; healthy controls Spearman r = 0.57, P < 0.0001].
Enthesis chronic abnormalities
The comparative analysis of the calcification score across the three groups and the bone erosion count is summarized in [Table - 3].

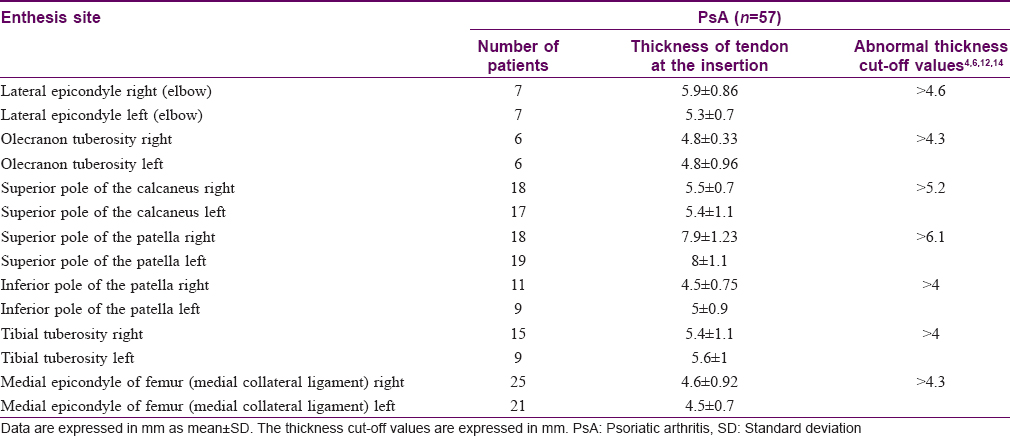
A sub-analysis conducted on psoriatic arthritis patients with erosive changes of entheses revealed that of the 8 entheses examined, erosive changes were observed mainly at the following sites: (i) medial epicondyle of femur, (ii) superior pole of the patella, and (iii) superior pole of the calcaneus.
It is important to note that the average thickness of the entheses affected by bone erosions increased compared to the normal values [Table - 4].
Discussion
Ultrasound has a relevant role in the diagnosis and management of psoriatic arthritis mainly owing to its capacity to diagnose clinically undetectable arthritis and enthesitis. Moreover, previous reports have indicated the usefulness of ultrasound in detecting signs of enthesitis in only skin psoriasis patients without clinical signs of inflammatory arthritis.[8],[9],[10],[13]
The purpose of our study was to clarify whether ultrasound measurement of enthesis thickness allows differentiation of patients with only skin psoriasis from psoriatic arthritis patients with asymptomatic enthesitis.
In our opinion, the thickness measurement represents the most reproducible method for assessing the inflammatory enthesitis. In fact, the power Doppler signal may not be detected even during the course of inflammation, especially in the very early stages of the disease. On the contrary, findings such as calcifications or erosions are expression of chronic inflammation and irreversible damage.[11],[12],[19]
Our analysis showed (for the first time), that the major differences in thickness between psoriasis cases with and without psoriatic arthritis were at the following enthesis sites: (i) olecranon tuberosity and (ii) superior pole of the patella [Table - 2]. Therefore, these two should be regarded as critical when scanning for enthesitis by ultrasound in psoriatic patients. Regarding the comparative analysis of the entheseal thickness between only skin psoriasis and healthy controls, we found statistically significant differences at the lateral and medial entheses of the elbow at the level of the superior pole of the patella and at the tibial insertion of the patellar tendon.
Our data is consistent with the hypothesis suggested by recent studies that a subclinical involvement of tendons and entheses is present in these patients.[10],[14]
From a histopathological viewpoint, the tendon thickening detected by ultrasound may be explained by the disorganization of the normal fibrillar architecture, which is already present in the very early stages of the disease, as reported in recent studies.[2]
Another novel finding of the present study was the measurement of the thickness of the medial collateral ligament at the site of the femoral origin. Although the clinical relevance of the above-mentioned site is emphasized by the fact that it is included in the Leeds score, it has never been investigated by ultrasound in either only skin psoriasis or psoriatic arthritis. Our ultrasound data shows that the thickness of enthesis at this site is increased in psoriatic arthritis, but not in only skin psoriasis and healthy controls [Table - 2]. It may be noted that this site was the only one where there were no thickness differences between only skin psoriasis and healthy controls [Table - 2]. The significance of this last finding is at present unknown.
The fact that the enthesis thickness correlates with BMI and the age of the patients implies that there are many factors which could influence the enthesis thickness; nevertheless, interestingly we found a strong correlation between the thickness total score and the number of erosions.
In particular, in psoriatic arthritis patients the highest enthesis thickness was seen in areas where bone erosions were also present. These findings together with the data of a correlation between the enthesis thickness and the degree of calcification may indicate that thickening is an alteration closely linked to damage of the enthesis [Figure - 1].
 |
| Figure 1: |
Once again, the medial epicondyle of femur was the commonest site of involvement in psoriatic arthritis because erosive changes were seen in 50% of the patients studied [Figure - 2].
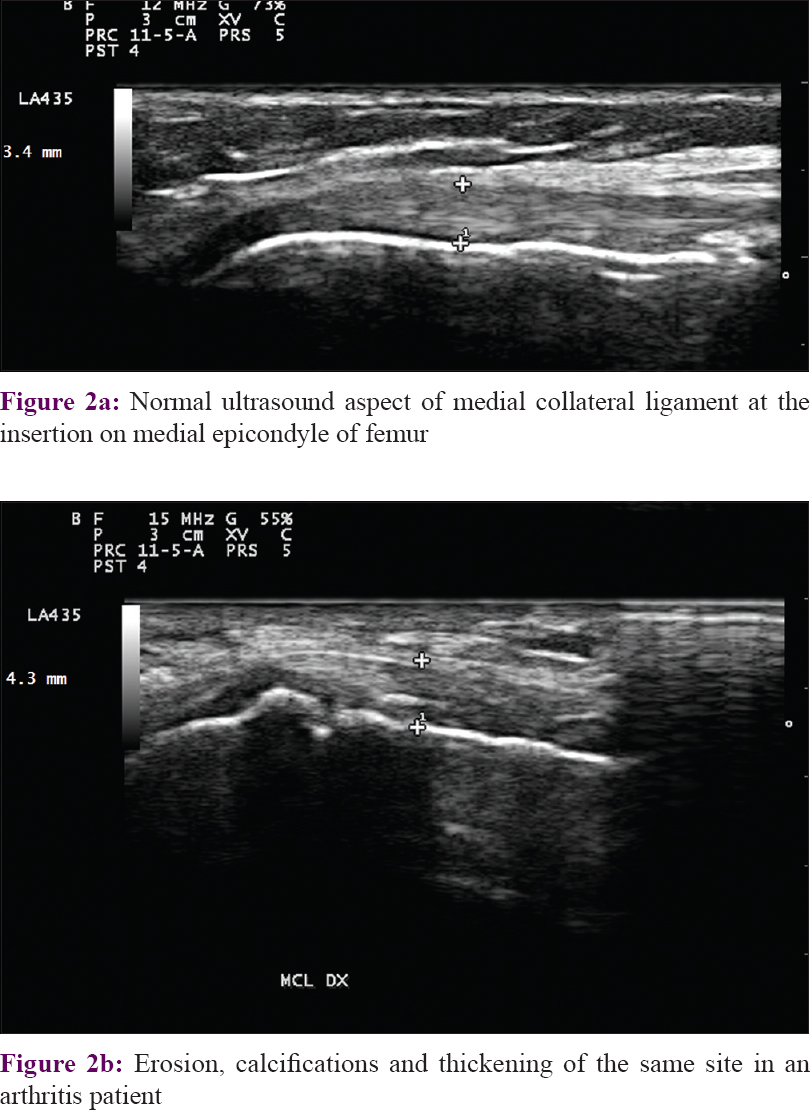 |
| Figure 2: |
The most important limitation of our study was the small sample of patients studied. In addition, the results do not include a comparative analysis with data from patients with clinically evident signs of enthesitis. This reinforces the need of longitudinal studies aimed to validate our preliminary observations.
Our results clearly demonstrate the existence of significant differences in the enthesis thickness between psoriatic arthritis and only skin psoriasis, even in sites never considered previously.
The present study confirms that the ultrasound measurement of enthesis thickness is a reliable and accurate method to evaluate in clinical practice; the degree of enthesis involvement in psoriasis patients especially in absence of clear signs of enthesitis detectable on clinical examination.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
McGonagle D, Khan MA, Marzo-Ortega H, O'Connor P, Gibbon W, Emery P. Enthesitis in spondyloarthropathy. Curr Opin Rheumatol 1999;11:244-50.
[Google Scholar]
|
| 2. |
McGonagle D, Marzo-Ortega H, O'Connor P, Gibbon W, Hawkey P, Henshaw K, et al. Histological assessment of the early enthesitis lesion in spondyloarthropathy. Ann Rheum Dis 2002;61:534-7.
[Google Scholar]
|
| 3. |
Ballanti E, Perricone C, di Muzio G, Kroegler B, Chimenti MS, Graceffa D, et al. Role of the complement system in rheumatoid arthritis and psoriatic arthritis: Relationship with anti-TNF inhibitors. Autoimmun Rev 2011;10:617-23.
[Google Scholar]
|
| 4. |
Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: Assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686-91.
[Google Scholar]
|
| 5. |
Heuft-Dorenbosch L, van Tubergen A, Spoorenberg A, Landewé R, Dougados M, Mielants H, et al. The influence of peripheral arthritis on disease activity in ankylosing spondylitis patients as measured with the bath ankylosing spondylitis disease activity index. Arthritis Rheum 2004;51:154-9.
[Google Scholar]
|
| 6. |
Gutierrez M, Zeiler M, Filippucci E, Salaffi F, Becciolini A, Bertolazzi C, et al. Sonographic subclinical entheseal involvement in dialysis patients. Clin Rheumatol 2011;30:907-13.
[Google Scholar]
|
| 7. |
Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis 2002;61:905-10.
[Google Scholar]
|
| 8. |
Naredo E, Möller I, de Miguel E, Batlle-Gualda E, Acebes C, Brito E, et al. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: A prospective case-control study. Rheumatology (Oxford) 2011;50:1838-48.
[Google Scholar]
|
| 9. |
de Miguel E, Cobo T, Muñoz-Fernández S, Naredo E, Usón J, Acebes JC, et al. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis 2009;68:169-74.
[Google Scholar]
|
| 10. |
Gisondi P, Tinazzi I, El-Dalati G, Gallo M, Biasi D, Barbara LM, et al. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: A hospital-based case-control study. Ann Rheum Dis 2008;67:26-30.
[Google Scholar]
|
| 11. |
Eder L, Jayakar J, Thavaneswaran A, Haddad A, Chandran V, Salonen D, et al. Is the MAdrid sonographic enthesitis index useful for differentiating psoriatic arthritis from psoriasis alone and healthy controls? J Rheumatol 2014;41:466-72.
[Google Scholar]
|
| 12. |
Mandl P, Niedermayer DS, Balint PV. Ultrasound for enthesitis: Handle with care! Ann Rheum Dis 2012;71:477-9.
[Google Scholar]
|
| 13. |
Gandjbakhch F, Terslev L, Joshua F, Wakefield RJ, Naredo E, D'Agostino MA, et al. Ultrasound in the evaluation of enthesitis: Status and perspectives. Arthritis Res Ther 2011;13:R188.
[Google Scholar]
|
| 14. |
Bandinelli F, Prignano F, Bonciani D, Bartoli F, Collaku L, Candelieri A, et al. Ultrasound detects occult entheseal involvement in early psoriatic arthritis independently of clinical features and psoriasis severity. Clin Exp Rheumatol 2013;31:219-24.
[Google Scholar]
|
| 15. |
Litinsky I, Balbir-Gurman A, Wollman J, Arad U, Paran D, Caspi D, et al. Ultrasound assessment of enthesis thickening in psoriatic arthritis patients treated with adalimumab compared to methotrexate. Clin Rheumatol 2016;35:363-70.
[Google Scholar]
|
| 16. |
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum 2006;54:2665-73.
[Google Scholar]
|
| 17. |
Lee JI, Song IS, Jung YB, Kim YG, Wang CH, Yu H, et al. Medial collateral ligament injuries of the knee: Ultrasonographic findings. J Ultrasound Med 1996;15:621-5.
[Google Scholar]
|
| 18. |
Toprak U, Başkan B, Üstüner E, Öten E, Altin L, Karademir MA, et al. Common extensor tendon thickness measurements at the radiocapitellar region in diagnosis of lateral elbow tendinopathy. Diagn Interv Radiol 2012;18:566-70.
[Google Scholar]
|
| 19. |
D'Agostino MA, Aegerter P, Bechara K, Salliot C, Judet O, Chimenti MS, et al. How to diagnose spondyloarthritis early? Accuracy of peripheral enthesitis detection by power Doppler ultrasonography. Ann Rheum Dis 2011;70:1433-40.
[Google Scholar]
|
Fulltext Views
5,975
PDF downloads
2,324





