Translate this page into:
Ultrathin split-thickness skin grafting followed by narrowband UVB therapy for stable vitiligo: An effective and cosmetically satisfying treatment option
Correspondence Address:
Imran Majid
Cutis Skin and Laser Institute, Karanagar Chowk, Srinagar, Kashmir
India
| How to cite this article: Majid I, Imran S. Ultrathin split-thickness skin grafting followed by narrowband UVB therapy for stable vitiligo: An effective and cosmetically satisfying treatment option. Indian J Dermatol Venereol Leprol 2012;78:159-164 |
Abstract
Background: Different surgical techniques in the form of tissue or cellular grafting procedures are used alone or in combination with narrowband UVB (NBUVB) to treat stable vitiligo resistant to medical treatment. Aim: To evaluate the cosmetic results obtained with ultrathin split-thickness skin grafts followed by NBUVB therapy in resistant, stable vitiligo. Methods: Forty patients of stable vitiligo were treated with ultrathin split-thickness grafting and the patients were then put on NBUVB therapy. The results obtained were analyzed by the extent of repigmentation achieved as well as the final cosmetic outcome at the recipient as well as donor sites. Results: The first evidence of repigmentation was seen in the second week after starting NBUVB. On objective assessment, more than 90% repigmentation was seen in 83% of patients and the overall cosmetic results at the recipient site were graded as good to excellent in 90% patients at the end of NBUVB treatment. Perigraft halo of depigmentation was seen in six patients (15%) on the recipient site. Hypertrophic scarring was observed in two patients at the donor site. Conclusions: Ultrathin split-thickness skin grafting, when combined with NBUVB therapy, leads to better cosmetic outcome with faster onset of repigmentation in resistant stable vitiligo.Introduction
Vitiligo is a common acquired disorder of pigmentation that causes a tremendous impact on the quality of life in affected patients. [1],[2] The disease affects about 1% of the world population and spares almost no racial or ethnic group. [3] The majority of patients affected with vitiligo are treated by medical means, and there still remains a group which is refractory to all these medical lines of treatment. Segmental vitiligo as well as vitiligo distributed over acral parts and non-hairy areas, such as the wrist, feet, and male genitals, respond poorly to medical therapies in general. [4] Vitiligo that is stable and refractory to medical therapies can be treated by certain surgical procedures like tissue or cellular grafting techniques. These surgical procedures basically donate some viable melanocytes to the affected area of depigmentation. These viable melanocytes are then stimulated by different means like PUVA or PUVAsol therapy, [5],[6],[7] narrowband UVB (NBUVB), [8],[9],[10] topical steroids, [11] and even excimer laser treatment [12] to cause melanin production and thereby causing a repigmentation of the recipient or treated area.
Grafting procedures used in vitiligo can be divided into two main groups-"tissue grafting" wherein epidermis with or without a part of the dermis is transplanted on to the affected depigmented area and "cellular grafting" wherein the tissue grafts taken are treated by certain chemicals to separate the different cellular components and then the desired cellular component is transplanted with or without further culture on to the recipient skin. Although tissue grafting procedures are easier and simpler to perform than cellular grafting, the latter can be used to treat larger areas of depigmentation in a single session. [13] As far as tissue grafting procedures are concerned, there is a wide choice in the form of miniature punch grafting, [14] suction blister grafting, [15] split-thickness skin grafting, [16],[17] and even follicular skin grafting. [18] Each of these surgical techniques has its own advantages as well as disadvantages. [13] Miniature punch grafting is the simplest procedure to perform, but it is commonly associated with adverse effects like "cobble-stoning" and "polka-dot" appearance at the recipient site as well as some residual scarring at the donor site. [19] Suction blister grafting is associated with an excellent cosmetic and color matching at the recipient site and it does not normally cause any scarring at the donor site as well, but the procedure is time consuming and can take care of only smaller areas of depigmentation in a single sitting. [20] Split-thickness skin grafting is another tissue-grafting procedure used in vitiligo which provides a good, cosmetically acceptable repigmentation at the recipient site and does not lead to significant scarring at the donor site as well. [16],[17],[21] The procedure is also not time consuming and can take care of a relatively larger area in a single session. [22] The only limitation of split-thickness skin grafting is that, it needs a properly trained person to perform the procedure, as the overall results depend the mostly on the quality of grafts obtained. With ultrathin grafts without any dermal component present, excellent results can be achieved at both the recipient as well as donor sites. The use of such ultrathin grafts has been described in vitiligo with or without supplemental ultraviolet exposure. [6],[23] Such grafts are even useful for special sites like the eyelids where the skin is normally very thin. [24]
Methods
This study was done on 40 patients suffering from stable vitiligo resistant to all medical lines of treatment. The patients were recruited over a period of about 1 year (2009-2010) from the regular outpatient consultation or from the group receiving NBUVB phototherapy with resistant residual lesions. Inclusion criteria included patients having segmental, focal, or residual vitiligo after medical treatment, stable for at least one year and not showing response to any medical treatment including phototherapy. Stability was defined as no progression of lesions already present, no fresh lesions, and no evidence of Koebner phenomenon over at least 1 year. [13] Patients with any contraindication to the surgical procedure or to the phototherapy regimen, patients having keloidal tendency, and patients with unrealistic expectations were excluded from this study. Ethical clearance was obtained and a written consent form was signed by every patient or his/her guardian before the procedure.
Split-thickness skin grafting was performed in single or multiple sessions depending upon the area of skin to be treated. The donor site chosen for harvesting the grafts was the anterior aspect of upper thigh in all patients. The whole procedure at the donor as well as the recipient sites was done under the influence of a topical anesthetic cream in almost all the patients. Patients feeling pain even after the application of topical anesthetic were given additional infiltration with 1% lignocaine injection. The topical anesthetic used was a combination of topical prilocaine and lignocaine in a cream base under occlusion (Prilox cream). The cream was applied on the donor as well as the recipient sites for 60 minutes before the procedure. To take ultrathin epidermal grafts from the donor area, the skin on the thigh was stretched both proximally as well as distally by applying a tangential force and epidermal grafts of uniform thickness were obtained using Silver′s knife. The grafts taken differed from the traditional split-thickness grafts as there was no or minimal dermal tissue on their undersurface. The quality of the grafts was gauged by means of their translucent nature and by their ability to float on the isotonic saline solution. Grafts showing any dermal component in the form of a whitish undersurface were discarded and only translucent grafts were used for the procedure. The grafts, thus taken, were suspended in a sterilized, isotonic saline solution.
The recipient area was first cleaned with povidone iodine lotion and then dermabraded using either manual dermabraders or a motorized dermabrading apparatus. Dermabrasion was performed 0.5 mm beyond the margin of the recipient area to prevent any perigraft halo of depigmentation. The ultrathin epidermal grafts suspended in isotonic saline were then spread onto the dermabraded recipient sites with the help of glass slides and graft spreaders. The recipient area was then covered with double layer of antibiotic (chlorhexidine) coated gauze dressings. Oral antibiotics were also administered for 1 week after the procedure. Saline-soaked gauze dressing was then applied over the same, and lastly, a pressure dressing was applied to secure the grafts. The donor site was also covered with double layer of antibiotic-coated gauze dressing followed by pressure dressing. The dressing at the recipient area was kept for a duration of 7 to 9 days and then the dressing was removed carefully to keep the grafts in place.
NBUVB therapy was started 3 to 5 days after removing the dressing and was given thrice a week on nonconsecutive days according to set protocol. The initial dose of NBUVB was 200 mJ/cm 2 in all cases and the dose increments were 20% of the previous dose till faint erythema or perifollicular pigmentation was seen. Any further increments in dose, whenever needed, were in the range of 10% of the previous dose received. NBUVB therapy was thus continued for a period ranging from 1 to 3 months, depending upon the response in an individual patient. The response in each of the patients was assessed every week for the first month and then every 2 weeks thereafter, both clinically as well as with repeated digital photographs. In those patients who required multiple sessions of split-thickness skin grafting at different sites of the body, NBUVB was interrupted for 1 week before each grafting session and restarted immediately after the removal of dressings. The grafting sessions in these patients were spaced at 4- to 12-week intervals. The patients were followed up for a period ranging from 3 to 12 months, depending upon their response to treatment as well as the total number of sessions performed. Some patients even needed more than desired sessions at multiple sites.
At the end of the study period, the response to treatment was evaluated objectively on the basis of the extent of repigmentation achieved as well as the cosmetic matching at the recipient site. Cosmetic outcome at the donor site was also taken into consideration. In addition, the time to onset of repigmentation and any adverse events to the overall procedure were also noted down.
Results
All 40 patients were available for evaluation and belonged to Fitzpatrick skin type 3 or 4. A total of 84 lesions were treated, which were distributed on different sites of the body as given in [Table - 1]. The patients had either a segmental, focal, or patchy vitiligo. No patient with palmar, plantar, or finger-tip vitiligo was treated in this study.

All patients tolerated the procedure well under the effect of topical anesthetic cream. No patient required any additional anesthesia at the donor site. However, some patients (22.5%) needed additional infiltration with injectable 1% lignocaine at the recipient site as they could not tolerate the dermabrasion procedure under topical anesthetic alone.
There were no adverse events to the phototherapy regimen.
Perigraft halo of depigmentation was the commonest complication of the surgical procedure, seen in 14 lesions in 15% of patients [Figure - 1]. Graft displacement was seen in patients who underwent the procedure on lateral side of the neck. Two patients developed hypertrophic scarring [Figure - 2] at the donor site. In both these patients, the graft taken from the thigh was of a nonuniform thickness. The graft in these patients was cut into small pieces and only the thinner, translucent areas of the graft were transplanted on the recipient area. There were no complications seen in any of these patients on the recipient area and the cosmetic results achieved at the recipient site were satisfactory. No patient in the study developed curling of the grafts or milia at the recipient site. Similarly, there was no scarring seen at the donor site in any other patient except those mentioned above. In fact, in patients requiring multiple sessions, we were able to take grafts from a single site repeatedly.
 |
| Figure 1: (a, b) Segmental vitiligo with some perigraft depigmentation |
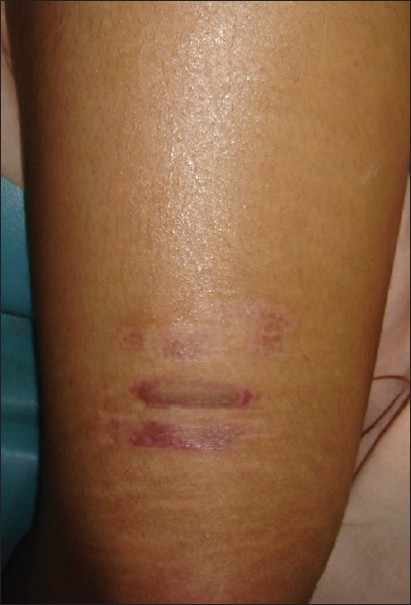 |
| Figure 2: Hypertrophic scarring on donor area secondary to a thicker graft |
The mean time to onset of repigmentation was 11 days (range, 7 to 18 days) after the start of NBUVB therapy. NBUVB was administered for a mean duration of 1.8 months (range, 1-month to 2.8 months).
About 83% (33 of 40) of patients achieved >90% repigmentation of the grafted lesions. Cosmetic matching was graded on visual analogue scale as good to excellent in 90% (36 of 40) cases.
As far as individual lesions were concerned, 70 lesions achieved >90% repigmentation in 33 patients [Table - 1]. Lesions on the face showed the best results, with all the 26 lesions in 18 patients achieving >90% repigmentation and a good cosmetic match [Figure - 3]. Lesions on the arms, abdomen, and back also showed really good results to the combination of partial thickness grafting with NBUVB. Twelve elbow lesions were transplanted in few patients, of which majority of lesions showed >90% repigmentation. Rest of the lesions showed a partial response only. Eyelid lesions were grafted in few patients and all of them responded quite well (with >90% repigmentation) to the treatment regimen. We had patients in whom the procedure was performed on the breasts and both these patients (lesions in total) showed >90% repigmentation and excellent cosmetic match at the treatment sites. Areola or nipples were not involved in any of these two patients.
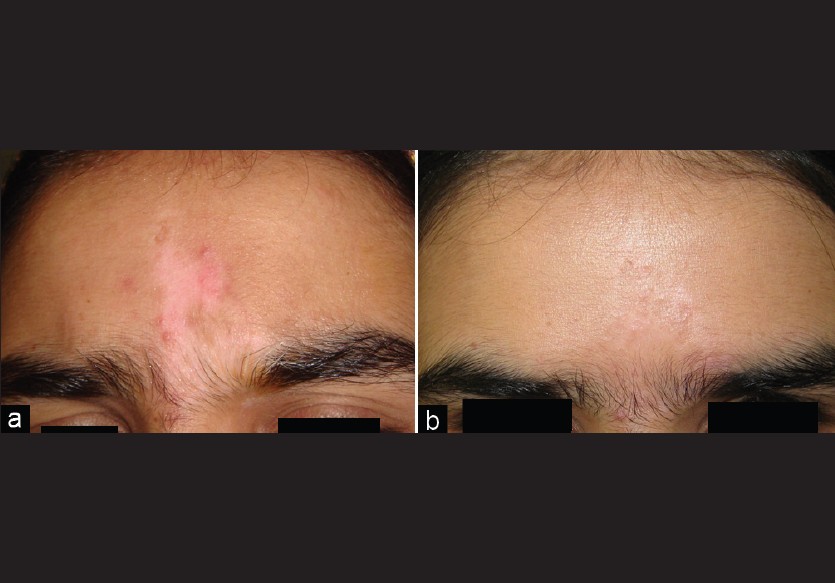 |
| Figure 3: (a, b) Stable, focal vitiligo on forehead with excellent cosmetic match |
On the feet, a few patients underwent transplantation and all of them responded with >90% repigmentation and an excellent color match. Even lesions on the ankle joints responded well to treatment. However, toe-tips or plantar skin was not involved in any of these patients.
Correlating the cosmetic results achieved with the site of the treated lesion, the face was seen to respond the best [Figure - 4]. Lesions on the elbows, ankles, and neck required special precautions to keep the grafts undisplaced.
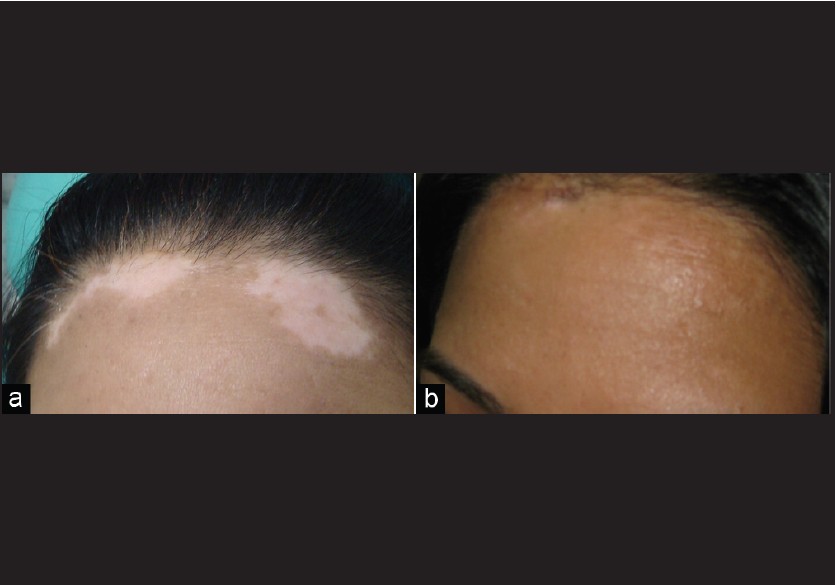 |
| Figure 4: (a, b) Residual vitiligo after NBUVB with good response to split-thickness skin grafting with NBUVB |
Discussion
Vitiligo that is stable and does not respond to medical lines of treatment can be managed by certain surgical procedures in the form of tissue or cellular grafting techniques. Any form of resistant vitiligo like focal or segmental vitiligo or even any residual lesions of patchy or generalized vitiligo can thus be treated satisfactorily in affected patients. Split thickness skin grafting, wherein a thin sheet of epidermis is taken from an appropriate donor area and then transplanted on the dermabraded vitiligo lesion, offers certain advantages over other tissue grafting procedures like mini-punch grafting and suction blister grafting. The cosmetic outcome achieved after split thickness skin grafting at the recipient as well as the donor site depend the most on the quality of the grafts taken. With ultrathin grafts having no accompanying dermal tissue, the quality of pigmentation achieved at the recipient site is usually excellent. By definition, a split-thickness graft is one that contains the full thickness of epidermis along with some portion of the papillary dermis. This dermal component is visible as a whitish undersurface in a normal split-thickness graft. The grafts that we used in the present study were all translucent grafts without any whitish tissue at the base. We laid emphasis on the translucent quality of the grafts obtained and not on the overall size of the grafts. Even if we had many small translucent grafts, they were used alongside each other on the recipient area. In the postoperative period, such grafts behave much like suction-blister grafts and even the color matching achieved is comparable with suction blister grafting [Figure - 3] and [Figure - 4]. With the use of ultrathin grafts, there are minimal chances of scarring at the donor site owing to the loss of epidermis alone from the area. We have observed that if one is able to achieve an ultrathin graft at the donor site, the same site can even be repeatedly used for taking similar grafts in the future. In fact, in our experience we have observed that it becomes easier to obtain a graft at the next session as the epidermis gets easily separated once it has regrown over the already-used donor area.
Grafting procedures are supplemented with NBUVB [8],[9],[10] or PUVA [5],[6],[7] to increase the chances of repigmentation as well as to achieve quicker results. NBUVB scores over PUVA as a treatment option in vitiligo as it is safer and does not require post-treatment protection from sun exposure. Another advantage of NBUVB is that it can be used safely in children as well. [25] NBUVB is thought to have a proliferative as well as a stimulatory effect on the melanocytes donated through the grafting procedure. Thus one can achieve a rapid repigmentation when it is used as a supplementary therapy along with any grafting procedure. NBUVB has been used in combination with mini-punch grafting with good results. [8] It has also been used after autologous epidermal cell culture procedure in resistant vitiligo with gratifying results. [9]
Clinical studies have shown that in case of split-thickness skin grafting, supplemental NBUVB not only causes a rapid repigmentation but also minimizes the chances of perigraft depigmentation. [24] Even excimer laser with its monochromatic 308 nm wavelength has been used in combination with split-thickness skin grafting and the authors have reported really good and early results with this combination. [12] What we have observed in our patient′s that if ultrathin split-thickness skin grafting is followed immediately by a course of NBUVB, the onset of repigmentation is quite rapid as we have been able to see an evidence of repigmentation as early as the second week after the start of NBUVB regimen. Moreover, the color match achieved at the recipient site is also improved.
Complications described in case of split-thickness skin grafting at the recipient site are hyperpigmentation, curling of the graft, milia formation, and lastly graft contracture leading to achromic fissures between the individual grafts or perigraft depigmentation at the margins. Similarly, there are chances of scarring and hyper- or hypo-pigmentation at the donor site. We did observe some perigraft depigmentation in our patients, but the other adverse effects were rarely seen in our study. This can possibly be ascribed to the use of ultrathin grafts in this study population. We used only those grafts that had no dermal tissue accompanying them and if there was a graft of non-uniform thickness, we either discarded the whole graft or used only the translucent portions of the graft. The low incidence of perigraft depigmentation can also be possibly ascribed to the beneficial effect offered by the concurrent NBUVB treatment.
Split-thickness skin grafting is claimed to be the most successful tissue-grafting procedure in vitiligo and this has been our observation as well. With the use of ultrathin skin grafts, the outcome at both the donor as well as the recipient sites can be improved further. The procedure, when performed in carefully selected patients with a proper technique, can certainly provide excellent cosmetic results in resistant stable vitiligo. Concurrent use of NBUVB therapy gives faster and better cosmetic results.
| 1. |
Mattoo SK, Handa S, Kaur I, Gupta N, Malhotra R. Psychiatric morbidity in vitiligo: Prevalence and correlates in India. J Eur Acad Dermatol Venereol 2002;16:573-8.
[Google Scholar]
|
| 2. |
Aghaei S, Sodaifi M, Jafari P, Mazharinia N, Finlay AY. DLQI scores in vitiligo: Reliability and validity of the Persian version. BMC Dermatol 2004;4:8.
[Google Scholar]
|
| 3. |
Kovacs SO. Vitiligo. J Am Acad Dermatol 1998;38:647-66.
[Google Scholar]
|
| 4. |
Njoo MD, Spuls PI, Bos JD, Westerhof W, Bossuyt PM. Nonsurgical repigmentation therapies in vitiligo: Meta-analysis of the literature. Arch Dermatol 1998;134:1532-40.
[Google Scholar]
|
| 5. |
Hann SK, Im S, Bong HW, Park YK. Treatment of stable vitiligo with autologous epidermal grafting and PUVA. J Am Acad Dermatol 1995;32:943-8.
[Google Scholar]
|
| 6. |
Awad SS, Abdel-Raof H, Hosam El-Din W, El-Domyati M. Epithelial grafting for vitiligo requires ultraviolet-A phototherapy to increase success rate. J Cosmet Dermatol 2007;6:119-24.
[Google Scholar]
|
| 7. |
Savant SS. Surgical therapy of vitiligo: Current status. Indian J Dermatol Venereol Leprol 2005;71:307-10.
[Google Scholar]
|
| 8. |
Lahiri K, Malakar S, Sarma N, Banerjee U. Repigmentation of vitiligo with punch grafting and narrow-band UV-B (311 nm) a prospective study. Int J Dermatol 2005;45:649-55.
[Google Scholar]
|
| 9. |
Lahiri K, Malakar S. Inducing repigmentation by regrafting and phototherapy (311 nm) in punch failure cases of lip vitiligo: A pilot study. Indian J Dermatol Venereol Leprol 2004;70:156-8.
[Google Scholar]
|
| 10. |
Elisa P, Massimiliano R, Andre A, Paolo T, Francesca I, Lucio A. Autologous epidermal cultures and narrow-band ultraviolet B in the surgical treatment of vitiligo. Dermatol Surg 2005;31:155-9.
[Google Scholar]
|
| 11. |
Barman KD, Khaitan BK, Verma KK. A comparative study of punch grafting followed by topical corticosteroid versus punch grafting followed by PUVA therapy in stable vitiligo. Dermatol Surg 2004;30:49-53.
[Google Scholar]
|
| 12. |
Al-Mutairi N, Manchanda Y, Al-Doukhi A, Al-Haddad A. Long-term results of split-skin grafting in combination with excimer laser for stable vitiligo. Dermatol Surg 2010;36:499-505.
[Google Scholar]
|
| 13. |
Parsad D, Gupta S. Standard guidelines of care for vitiligo surgery. Indian J Dermatol Venereol Leprol 2008;74:37-45.
[Google Scholar]
|
| 14. |
Falabella R. Repigmentation of stable Leukoderma by autologous mini-grafting. J Dermatol Surg Oncol 1986;12:172-9.
[Google Scholar]
|
| 15. |
Gupta S, Shroff S, Gupta S. Modified technique of suction blistering of epidermal grafting in vitiligo. Int J Dermatol 1999;38:306-9.
[Google Scholar]
|
| 16. |
Kahn AM, Cohen MJ. Repigmentation in vitiligo patients-melancocytes transfer via ultra-thin grafts. Dermatol Surg 1998;24:365-7.
[Google Scholar]
|
| 17. |
Achauer BM, Le Y, Vander Kam VM. Treatment of vitiligo with melanocytic grafting. Ann Plast Surg 1994;33:644-6.
[Google Scholar]
|
| 18. |
Na GY, Seo SK, Choi SK. Single hair grafting for the treatment of vitiligo. J Am Acad Dermatol 1998;38:580-4.
[Google Scholar]
|
| 19. |
Babu A, Thappa DM, Jaisankar TJ. Punch grafting versus suction blister epidermal grafting in the treatment of stable lip vitiligo. Dermatol Surg 2008;34:166-78.
[Google Scholar]
|
| 20. |
Falabella R. Surgical therapies for vitiligo and other leukodermas, part 1: Minigrafting and suction epidermal grafting. Dermatol Ther 2001;14:7-14.
[Google Scholar]
|
| 21. |
Agrawal K, Agrawal A. Vitiligo: Repigmentation with dermabrasion and thin split-thickness skin graft. Dermatol Surg 1995;21:295-300.
[Google Scholar]
|
| 22. |
Njoo MD, Westerhof W, Bos JD, Bossuyt PM. A systematic review of autologous transplantation methods in vitiligo. Arch Dermatol 1998;134:1543-9.
[Google Scholar]
|
| 23. |
Olsson M, Juhlin L. Long-term follow-up of leukoderma patients treated with transplants of autologous cultured melanocytes, ultra-thin epidermal sheets and basal cell layer suspension. Br J Dermatol 2002;47:893-904.
[Google Scholar]
|
| 24. |
Khunger N, Kathuria SD, Ramesh V. Tissue grafts in vitiligo surgery - Past, present, and future. Indian J Dermatol 2009;54:150-8.
[Google Scholar]
|
| 25. |
Njoo MD, Boss JD, Westerhof W. Treatment of generalised vitiligo in children with narrow-band (TL-01) UVB radiation therapy. J Am Acad Dermatol 2000;42:245-53.
[Google Scholar]
|
Fulltext Views
8,503
PDF downloads
3,516





