Translate this page into:
Variable therapeutic outcomes with baricitinib in refractory generalised granuloma annulare - Need for type specific cytokine data
Corresponding author: Dr. Ananta Khurana, Department of Dermatology, Atal Bihari Vajpayee Institute of Medical Sciences and Dr. Ram Manohar Lohia Hospital, Delhi, India. drananta2014@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Khurana A, Sharath S, Bansal A, Sardana K, Kathirvelu AP, Muddebihal A. Variable therapeutic outcomes with baricitinib in refractory generalised granuloma annulare - Need for type specific cytokine data. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1511_2024
Dear Editor,
Granuloma annulare (GA) is an idiopathic granulomatous inflammatory skin disorder that presents as erythematous papules and annular plaques in elderly females. Skin lesions are localised in most patients and self-resolving. However, around 15% of patients have generalised (>10) lesions that are usually treatment unresponsive.1 Various treatment modalities have been used like steroids, psoralen plus ultraviolet A (PUVA), isotretinoin, dapsone, pentoxifylline, hydroxychloroquine, cyclosporine, interferon-gamma (IFN-γ), potassium iodide, nicotinamide, niacinamide, salicylic acid, dipyridamole, fumaric acid ester, etanercept, infliximab, adalimumab but with limited evidence.2
Understanding of the pathogenetic mechanisms of GA is insufficient, which precludes the use of targeted therapy. With evidence from recent immunological studies suggesting the role of Janus kinase (JAK) pathways and related cytokines, JAK inhibitors (JAKi) such as abrocitinib, tofacitinib, baricitinib, and upadacitinib have been successfully used in the treatment of GA.1,3-5 We report outcomes of treatment with baricitinib in three patients with generalised GA, one having undergone lesional cytokine mRNA analysis. We retrospectively analysed records of three patients who were diagnosed with refractory generalised GA with the diagnosis confirmed histologically and were treated with baricitinib monotherapy (4 mg orally once daily). Lesional and non-lesional skin biopsies were also sent for cytokine (mRNA levels) analysis before initiation of baricitinib in patient 1 and were analysed as per previously defined methods.6 The comparison between lesional and non-lesional skin showed upregulation of IL-21 (2.33-fold) and downregulation of IFN-γ (0.08-fold) and IL-15 (0.96-fold) in the former. Clinical and therapeutic details of patients are elaborated in Table 1.
| Cases | Age/sex | Comorbidities | Type and duration of GA | Previous treatments | Response | Adverse effects | Relapse |
|---|---|---|---|---|---|---|---|
| Patient 1 | 48 years/F | Type 2 diabetes mellitus, dyslipidemia, rheumatoid nodules | Generalised plaque GA, 6 years | HCQ 200 mg BD and potent TCS for 6 months with no improvement | GA plaques showed improvement starting at 6 weeks with 100% resolution of the plaques on hand and 90% improvement in plaques on feet at 14 weeks of treatment after which baricitinib was stopped. Rheumatoid nodules started to settle at 4 weeks with complete remission at 6 weeks. | Fever, sore throat, gastrointestinal disturbance and insomnia | Plaques on feet, and the nodules on hand relapsed after one month of remission |
| Patient 2 | 60 years/F | Type 2 diabetes | Generalised plaque, one year | Potent TCS and dapsone for 6 months with no improvement | Improvement in erythema and flattening of plaques by 8 weeks of treatment. Only 50% flattening of plaques was obtained by 12 weeks of baricitinib therapy, hence the drug was stopped at this time point. | None | - |
| Patient 3 | 59 years/F | None | Generalised papular GA [Figure 1], one year | Oral steroids on two occasions with some improvement but each time she suffered a quick relapse on stopping oral steroids. | Improvement was noted within 4 weeks of initiation of treatment and complete resolution of papules was achieved within 6 weeks of baricitinib [Figure 1]. | None | No new lesions till date (5 months) post-treatment discontinuation |
GA: Granuloma annulare; HCQ: hydroxychloroquine; BD: bis in die (twice daily); TCS: Topical corticosteroids
Baricitinib monotherapy gave a favourable response in the treatment of three patients with generalised GA for whom conventional treatment had previously failed. Response was noted as early as within four weeks of treatment. The best response was seen in disseminated papular GA, which responded within a short span of time [Figure 1]. The plaque variant took longer to respond (12-14 weeks vs six weeks) and did not show complete clearance in one patient. Relapse post discontinuation was observed in patient 1 with generalised plaques, while no relapse was observed in generalised papular GA patient (patient 3).
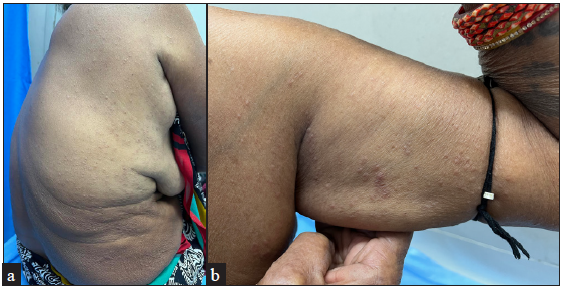
- Generalised erythematous papules on the trunk and limbs of Patient 1, suggestive of generalised papular GA.
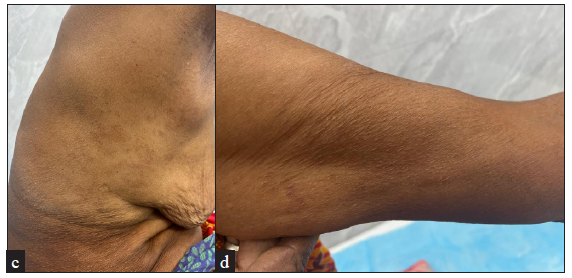
- Complete resolution of papules at 8-weeks of baricitinib.
IFN-γ, a crucial cytokine in the development of GA, as well as other molecular mediators - oncostatin M (OSM), IL-15, and IL-21 operate via the JAK-STAT pathway.5 GA is believed to be a T-cell dependent cutaneous inflammatory disorder involving T helper 1 (Th1) and Th2 polarisation, but the literature on Th17 or T regulatory (Treg) cytokine profiles is still inadequate. Recent RNA sequencing data, however, suggests upregulation of only Th1 cytokines in GA lesions, predominantly IFN-γ and IL-21.5 IFN-γ signals via JAK 1, JAK2, and TYK-2, while IL-21 signals via JAK1/JAK3 thus making GA amenable to treatment with JAK inhibitors [Table 2].1,3-5,7 In patient 1, a two-fold increase was observed in IL-21 levels (in lesional versus non-lesional skin) but none in IFN-γ, suggesting likely differences in cytokine responses between patients, possibly translating to varied clinical response.
| Case report | Age (years) /sex | Clinical type (duration) | Co-morbidities | Previous treatment | Treatment- dose and duration | Response | Relapse | Adverse effects |
|---|---|---|---|---|---|---|---|---|
| Yan et al., 20221 | 67/M | Generalised plaque GA (6 months) | Nil |
Oral HCQ 400 mg/day TCS NB UVB 3 times/week for 12 weeks |
Baricitinib 4 mg OD for 5 months | Plaques begin to clear at 2 months and almost cleared at 5 months | No relapse 4 months after stopping baricitinib | None |
| Jadoul et al., 20233 | 66/F | Generalised plaque GA | Obesity, hypertension, multinodular goitre, and haemochromatosis |
Over a period of 28 months, TCS, UVB therapy (22 sessions), cryotherapy, Methotrexate (15 mg weekly), cyclosporine (3 mg/kg/day), and adalimumab (40 mg biweekly) |
Baricitinib 4 mg OD | Rapid improvement in papules after 6 weeks of initiation. | Nil 8 months post-treatment | Herpes zoster at 7 weeks |
| Kim et al., 20234 | 67/F | Generalised papules and plaques GA (6 years) | Type 2 diabetes, hypertension | Methotrexate, ciclosporin, NBUVB phototherapy, topical tacrolimus for 4 months | Baricitinib 4 mg OD | Dramatic response within 6 weeks | Relapse 3 days after stopping | None |
| 40/M | Generalised papular GA (10 years) | None | Immunosuppressants and topical tacrolimus | Baricitinib 4 mg OD | Near complete remission in 1 month | On maintenance dose 4 mg | None | |
| Wang et al., 20215 | 53-68 years | Severe long-standing GA (plaques and papules) (6-15 years) | NA | Topical and oral corticosteroids, HCQ, cyclosporine, pentoxifylline, oral antibiotics, and phototherapy | Tofacitinib 5 mg BD for 6 months | At 6 months, 3 patients achieved complete remission and 2 showed marked response. | NA | Uncomplicated urinary tract infection |
GA: Granuloma annulare; HCQ: hydroxychloroquine; OD: omne in die (once a day); BD: bis in die (twice daily); TCS: Topical corticosteroids; NB UVB: Narrowband ultraviolet B; PUVA: Psoralen ultraviolet A, NA: Not available
There is little data on type-specific GA polarisation.However, while Wang et al.5 reported greater suppression of pathogenic cytokine activity in complete responders to tofacitinib versus partial responders, with increased IFN-γ, IL-21, IL-15, and OSM, we did not find significantly high IFN-γ activity, while IL-21 was raised twice above the normal skin level. However, while we compared the levels with the patient’s uninvolved skin, Wang et al. compared levels with normal skin biopsies from healthy controls.5 The lack of an IFN- γ signal may suggest a lower likelihood of response to baricitinib. Tofacitinib, which inhibits JAK1/JAK3 (and thus IL-21 signalling) may be a better option in such cases.
Baricitinib was well tolerated in our patients with only minor side effects. Lack of a consistent response in our series suggests that there is a need for tissue-based cytokine studies across the varied CD4 T cell profiles to effectively individualise treatment in this refractory disorder.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Successful treatment of generalized granuloma annulare with baricitinib. J Eur Acad Dermatol Venereol. 2022;36:e500-2.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of generalized granuloma annulare - a systematic review. J Eur Acad Dermatol Venereol. 2015;29:1467-80.
- [CrossRef] [PubMed] [Google Scholar]
- JAK1/2 pathway-specific treatment of disseminated granuloma annulare with baricitinib. J Eur Acad Dermatol Venereol. 2023;37:e1006-8.
- [Google Scholar]
- Rapid improvement of refractory generalized granuloma annulare with the Janus kinase inhibitor baricitinib in two patients. Clin Exp Dermatol. 2023;48:375-6.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-809.
- [CrossRef] [PubMed] [Google Scholar]
- Transcriptional upregulation of Nrf2-dependent phase II detoxification genes in the involved epidermis of vitiligo vulgaris. J Invest Dermatol. 2010;130:2781-9.
- [CrossRef] [PubMed] [Google Scholar]
- JAK inhibitors in dermatology: The road travelled and path ahead, a narrative review. Expert Rev Clin Pharmacol. 2023;16:279-95.
- [CrossRef] [PubMed] [Google Scholar]





