Translate this page into:
Verrucous dermatophytosis on photoexposed areas resembling pellagra in acquired immunodeficiency syndrome
2 Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Dipankar De
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh - 160 012
India
| How to cite this article: Bishnoi A, Ashraf R, Banerjee N, Saikia UN, De D. Verrucous dermatophytosis on photoexposed areas resembling pellagra in acquired immunodeficiency syndrome. Indian J Dermatol Venereol Leprol 2019;85:499-502 |
Sir,
A woman in her 40s with acquired immunodeficiency syndrome (AIDS)–associated cachexia presented for evaluation of multiple, well-defined, dark gray to black, polycyclic arciform plaques with a rugose surface and an erythematous rim on her face, V-area of neck and upper back [Figure - 1]a and [Figure - 1]b. Plaques on the face had a malar distribution and were also extending till the lower face and chin with well-defined areas of spared skin in between. Nasolabial folds were involved. In addition, she also suffered from severe herpes labialis and oral mucositis. Dorsa of hands and feet were not involved. She denied having pruritus, but had significant photosensitivity and complained of burning on exposure to sun light. She denied applying any topical medications prior to this presentation. Rubbing with alcohol swab did not clear the lesions.
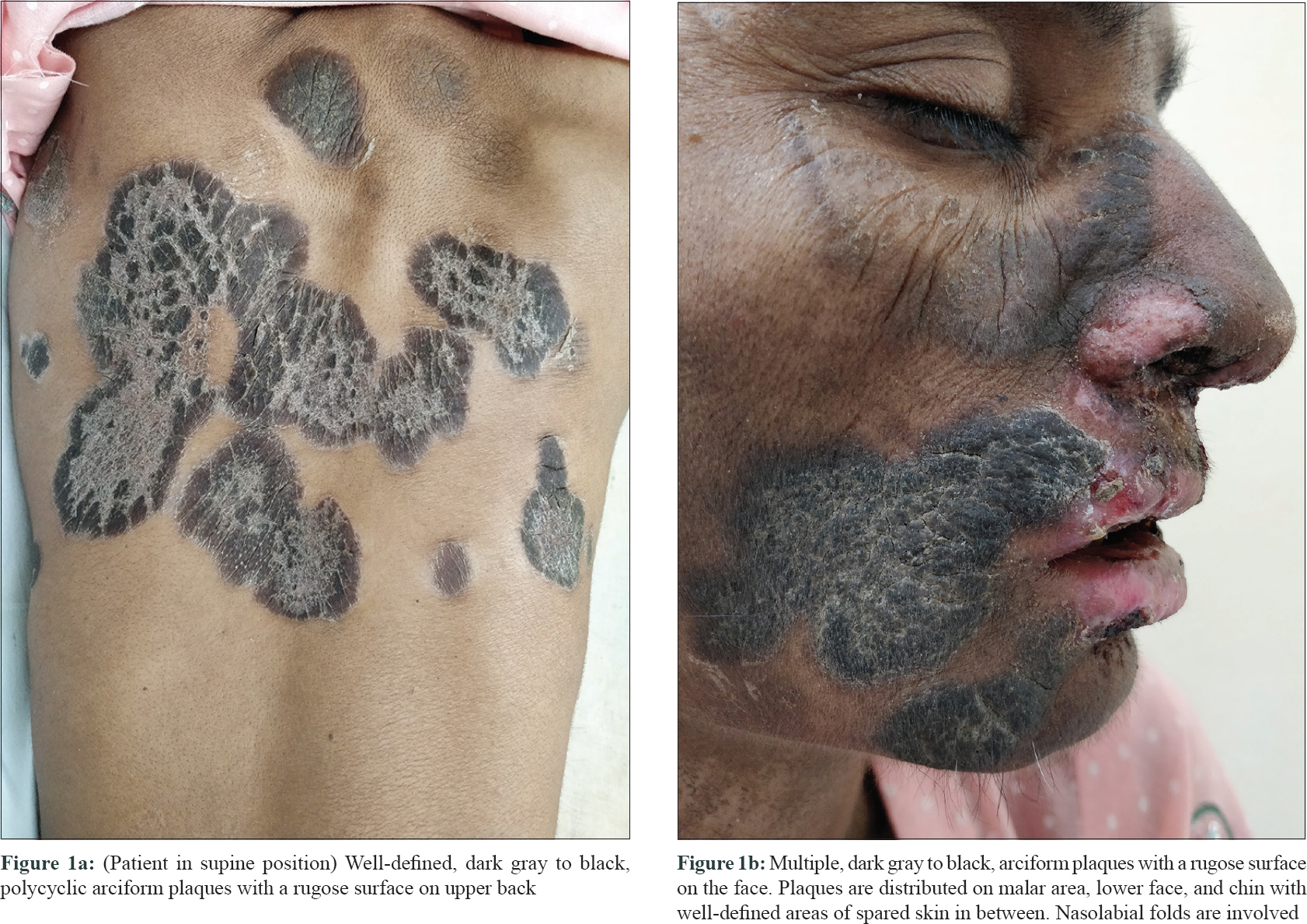 |
| Figure 1 |
Her CD4 count was 12/mm3. She also complained of loose stools and slight mental confusion, though she was cooperative and well-oriented to surroundings. Provisional diagnoses of pellagra and lupus erythematosus were considered and a skin biopsy was obtained from a lesion on the upper back. She was prescribed oral acyclovir and niacin 500 mg/day in addition to photoprotection. She had also been prescribed daily fluconazole, cotrimoxazole, atazanavir and ritonavir from the immunodeficiency clinic.
Interestingly, on her follow-up after a fortnight, the velvety hyperpigmented plaques on the upper back and neck were replaced by polycyclic plaques with raised rims, peripheral scaling and central clearing, precisely conforming to the margins of the previous plaques. These lesions were clinically suggestive of dermatophytosis [Figure - 2] and the change in morphology was attributed to the intake of fluconazole prescribed from immunodeficiency clinic (for oropharyngeal candidiasis, in a dose of 100 mg/day). Potassium hydroxide mount from the skin lesions at this time revealed abundant branching fungal hyphae. The histopathology of the skin biopsy that had been obtained on her first visit revealed hyperkeratosis, parakeratosis, mild papillomatosis and a slightly atrophic epidermis. On higher power, numerous fungal hyphae and spores suggestive of dermatophytes were seen in stratum corneum. Periodic acid–Schiff staining confirmed the presence of dermatophytic colonies in the stratum corneum [Figure - 3]a and [Figure - 3]b. There were no features suggestive of pellagra or lupus erythematosus in histopathology.
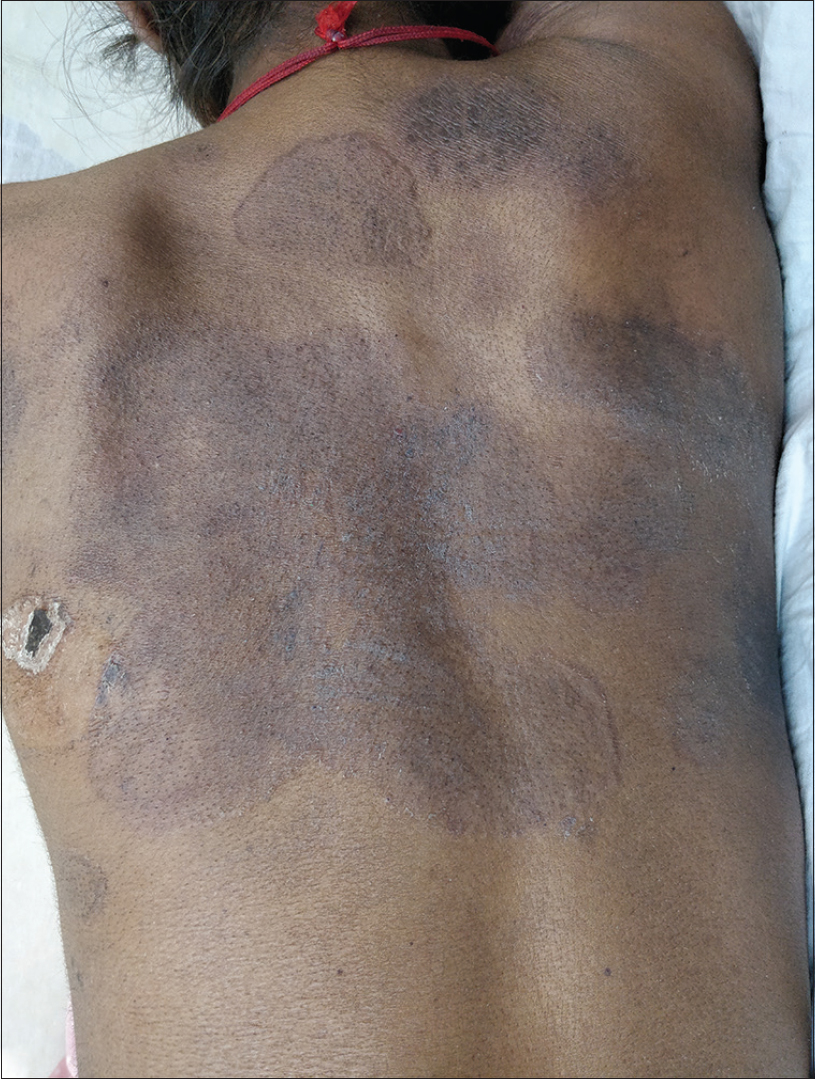 |
| Figure 2: (Patient in supine position) First follow-up visit, 2 weeks after baseline; polycyclic plaques with raised rims, peripheral scaling, and central clearing, precisely conforming to the margins of the previous plaques |
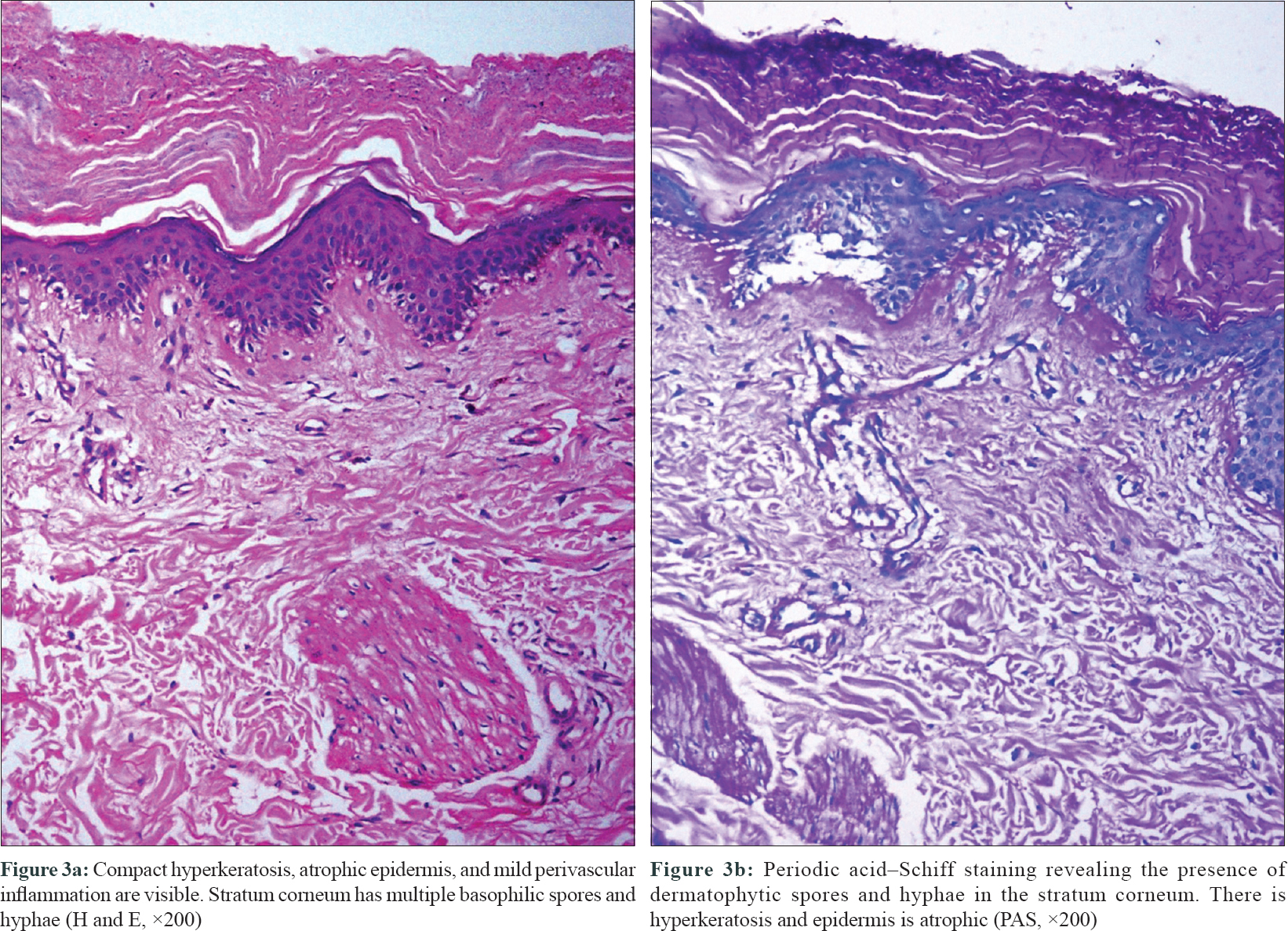 |
| Figure 3 |
Her antinuclear antibody titer was negative. There was no clinical evidence of onychomycosis. The lesions of orolabial herpes had healed. There was minimal improvement in the lesions of oropharyngeal candidiasis and therefore fluconazole was replaced with capsule itraconazole 100 mg twice a day (in consultation with immunodeficiency clinic). A skin scraping was sent for fungal culture and antifungal sensitivity at this visit.
Fungal culture of the skin scrapings revealed Trichophyton rubrum (susceptible to terbinafine and itraconazole, resistant to fluconazole). The patient reported after 6 weeks and the dermatophytic lesions and candidiasis had resolved completely [Figure - 4]a and [Figure - 4]b.
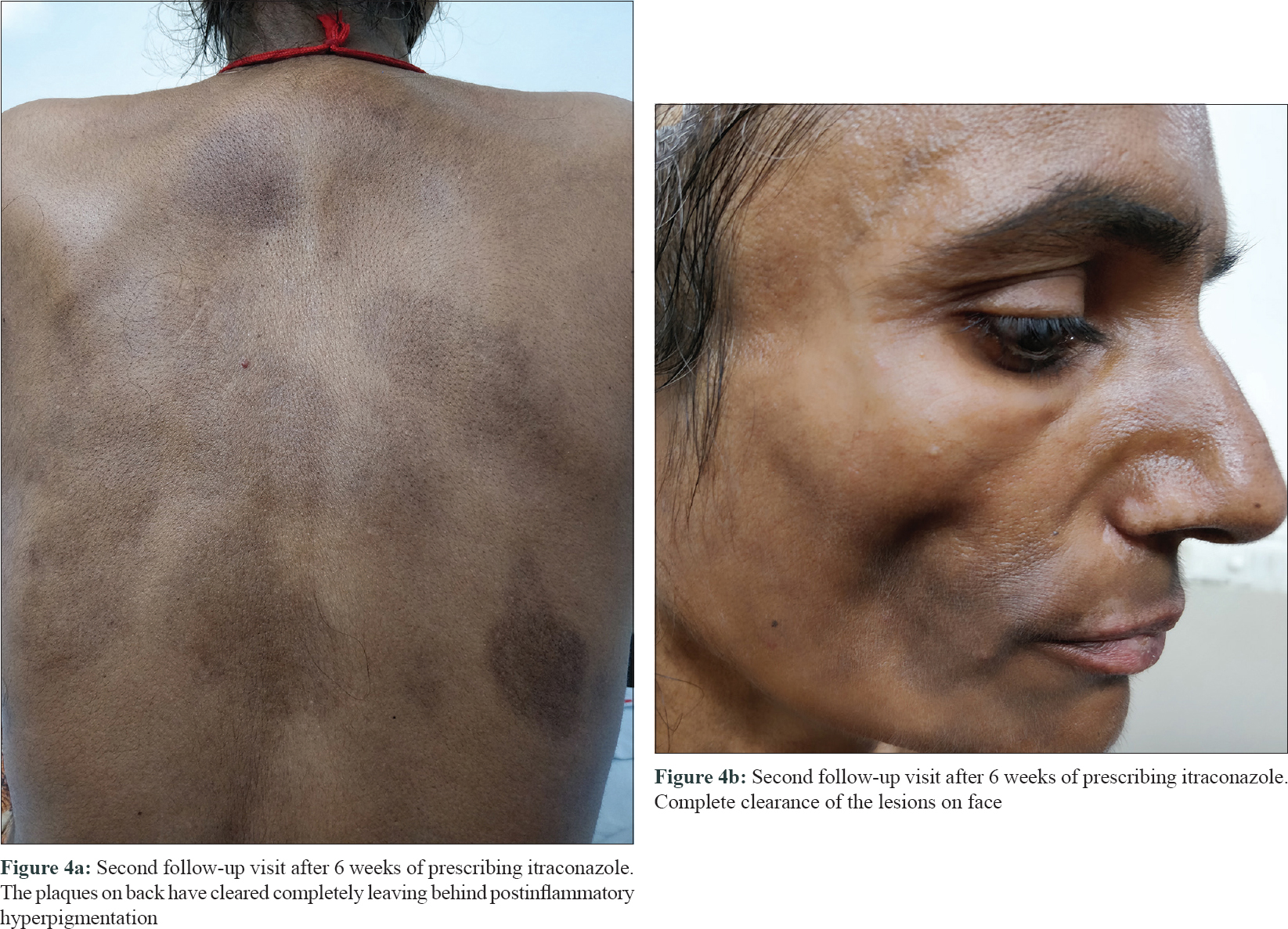 |
| Figure 4 |
Owing to the deterioration of the cellular immunity, AIDS is characterized by atypical morphological patterns of cutaneous viral infections (larger, chronic, necrotic, verrucous, hyperkeratotic, ecthymatous, and ulcerative lesions). The prevalence of dermatophytosis in the setting of human immunodeficiency virus infection has been reported variably in different studies. It is suggested that the occurrence of dermatophytosis correlates better with the viral load rather than CD4 cell counts.[1],[2] “Anergic tinea” with large, sharply marginated areas of hyperkeratosis, lacking a raised border and central clearance typical of tinea corporis, has been previously described in advanced human immunodeficiency virus infection.[3]
Photosensitivity occurs in 5% of people with human immunodeficiency virus infection and is attributed to a relative increase in the number of CD8+ T lymphocytes; deficiency of glutathione, thioredoxin, and tryptophan due to malnutrition and cachexia coupled with an accelerated metabolism; and concomitant administration of photosensitizing drugs (cotrimoxazole, etc.) in advanced human immunodeficiency virus infection/AIDS (CD4 counts below 50/mm3).[4]
”Tinea atypia” is being frequently encountered by dermatologists nowadays, in both immunosuppressed and immunocompetent individuals. There are reports in literature where at the time of presentation, dermatophytosis simulated other dermatoses such as psoriasis, granuloma annulare, eczema, impetigo, purpura, rosacea, seborrheic dermatitis, dermatitis herpetiformis, erythema multiforme and polymorphous light eruptions.[5],[6],[7] Reports also describe the localization of dermatophytosis on photoexposed sites that simulated lupus erythematosus.[5] Photodistribution in dermatophytosis, as also seen in the index patient, is interesting and needs to be studied further. To conclude, dermatophytosis should be thought of when photodistributed atypical verrucous polycyclic plaques present in immunocompromised individuals and it is prudent to obtain a potassium hydroxide mount from such lesions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
da Silva BC, Paula CR, Auler ME, Ruiz Lda S, Dos Santos JI, Yoshioka MC, et al. Dermatophytosis and immunovirological status of HIV-infected and AIDS patients from Sao Paulo city, Brazil. Mycoses 2014;57:371-6.
[Google Scholar]
|
| 2. |
Costa JE, Neves RP, Delgado MM, Lima-Neto RG, Morais VM, Coêlho MR, et al. Dermatophytosis in patients with human immunodeficiency virus infection: Clinical aspects and etiologic agents. Acta Trop 2015;150:111-5.
[Google Scholar]
|
| 3. |
Kaviarasan PK, Jaisankar TJ, Thappa DM, Sujatha S. Clinical variations in dermatophytosis in HIV infected patients. Indian J Dermatol Venereol Leprol 2002;68:213-6.
[Google Scholar]
|
| 4. |
Bilu D, Mamelak AJ, Nguyen RH, Queiroz PC, Kowalski J, Morison WL, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed 2004;20:175-83.
[Google Scholar]
|
| 5. |
Atzori L, Pau M, Aste N, Aste N. Dermatophyte infections mimicking other skin diseases: A 154-person case survey of tinea atypica in the district of Cagliari (Italy). Int J Dermatol 2012;51:410-5.
[Google Scholar]
|
| 6. |
Ziemer M, Seyfarth F, Elsner P, Hipler UC. Atypical manifestations of tinea corporis. Mycoses 2007;50 Suppl 2:31-5.
[Google Scholar]
|
| 7. |
D'Antuono A, Bardazzi F, Andalou F. Unusual manifestations of dermatophytoses. Int J Dermatol 2001;40:164-6.
[Google Scholar]
|
Fulltext Views
3,765
PDF downloads
2,655





