Translate this page into:
Virtual clinical trials–Implications for future dermatology research
Corresponding author: Dr. Gopikrishnan Anjaneyan, Department of Dermatology, Amrita Institute of Medical Sciences, Amrita Vishwa Vidyapeetham, Ponekkara, Kochi, Kerala, India. drgopikrishnana@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Anjaneyan G, Kaliyadan F, Pandhi D, Sankar R. Virtual clinical trials–Implications for future dermatology research. Indian J Dermatol Venereol Leprol. 2024;90:636-9. doi: 10.25259/IJDVL_1379_2023
Introduction
Clinical trials (CT), which form the scientific basis and fulcrum of drug development, are time-consuming and tedious. For a drug to reach the bedside from the bench, it takes approximately ten years with a humongous sum of money spent on it.1,2 Between money spent and the time consumed; the latter is the one that primarily drives the drug development costs.3 It is estimated that more than 70% of drugs fail to survive in the initial phases I and II of CTs. Less than 50% of the drugs that reach Phase III will eventually get regulatory approval.2 Despite these drawbacks, the number of CTs has increased exponentially over the past few years, with 2020 recording around 5000 of them, despite the COVID pandemic. According to the International Federation of Pharmaceutical Manufacturers & Associations, more than 8,000 compounds were at different stages of development globally and in 2020, the numbers of drugs in development were: oncology (2,740), immunology (1,535), neurology (1,498) and infectious diseases (1,213). In 2018 alone, the biopharmaceutical industry is estimated to have spent nearly 180 billion dollars globally on research and development.4
With imminent threats and inadvertent situations like the recent COVID pandemic being a deterrent for various ongoing and planned clinical research and trials, it is only natural to see that the future is moving towards a ‘virtual’ clinical trial system.
Patient recruitment has been one of the biggest stumbling blocks for CTs worldwide, primarily because of logistic difficulties and the enormous costs involved. At least 90% of trials are extended because investigators cannot recruit participants in a time-bound manner, and a mere 40% of the trial centres involved in a multi-centric study manage to recruit the required number. So the consequence is that the trials are longer, more expensive and often unfinished.2 The advent of telemedicine allows patients to connect to physicians by telephone or video for virtual visits, facilitating greater participation. There is a decreased burden on the participants and increasing consistency in record keeping.3 In the long run, this will make CTs more cost-effective by reducing costs related to travel and accommodation for participants and investigators.
Virtual Clinical Trials (VCT)
VCT, or decentralised trials, is a new and probably underutilised method of doing clinical research using advanced technologies (including dedicated apps and electronic monitoring devices) along with the help of electronic and online media [Figure 1].5 Trial decentralisation involves bringing an increasing proportion of a CT’s activities to the patient’s doorstep rather than using the conventional concept of bringing patients to a trial site.6 The study participants’ physical presence at the trial sites is minimised or eliminated. [Table 1].
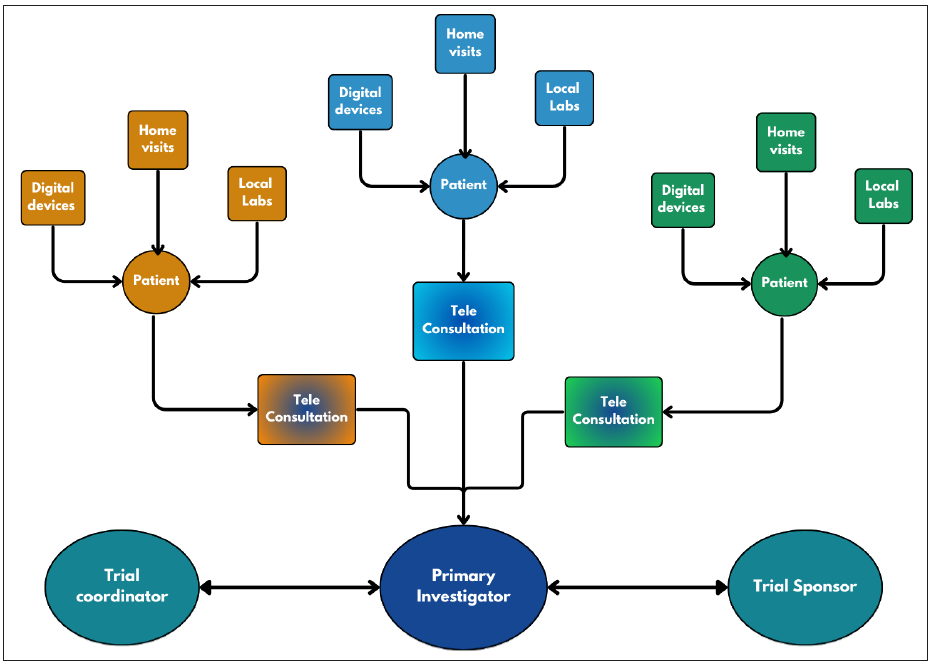
- Work-flow diagram of a virtual clinical trial.
| Features | Conventional clinical trials | Virtual clinical trials |
|---|---|---|
| Screening and recruitment | Phone calls and questionnaires | Electronic media – e questionnaires |
| In-person visits | Frequent site visits | Limited or nil visits |
| Broader recruitment from diverse backgrounds | Difficult | Easier |
| Generalisable results and representative of the real world | Less | More |
| Participant-centric | Less | More |
| History taking and consent | Trial site | Electronic media – e consent |
| Examination | Trial site | Local clinic/teleconsultation/home visit |
| Laboratory testing | Trial site | Local lab/home visit |
| Medications | Trial site | Home visit/local pharmacy |
| Cost involved | More | Less |
| Time for completion | More | Less |
First VCT
The REMOTE trial, which tested tolterodine tartrate (Detrol LA) for overactive bladder, was the world’s first completely virtual trial conducted by Pfizer. They studied whether the results of the pilot VCT can replicate the results of a previously completed Phase IV trial. Participants were recruited and managed remotely without visits to the trial site and entirely using web-based resources. The study concluded that efficacy was comparable with results from the original conventional trial. It helped simplify the multi-step screening, recruiting and testing process with VCT, ultimately providing a participant-friendly approach.7
Strengths of VCT vs conventional CTs
Impact on recruitment
Virtual visits help overcome frequent in-person participant visits and the associated time and travel expenditures. It could also simplify the participation of differently abled individuals, who would find it challenging to participate otherwise in conventional CTs.3
Participant centric
VCTs help reach a more diverse population and are more representative of the real world than conventional CTs. A broad recruitment strategy allows even patients far from the trial sites to participate in VCTs, and working individuals need not spend time travelling. It also ensures greater autonomy for the participant and is more convenient for the family members.8
Financial aspect
From a financial aspect, VCTs are also very useful – shorter enrolment periods and faster data collection, no travel expenses, and reimbursement compared to conventional trials. While conventional trials rely on clinical establishments and telephone calls for patient recruitment and follow-up (too dependent on predominantly the local population), virtual trials can use electronic media and questionnaires like Google Forms to target a wider population. This can cut down in-person participant visits, laboratory investigations, and subsequent treatment, which can be coordinated with the help of medical professionals at a local centre in the patient’s vicinity. This ensures savings of time and money and, in turn, better retention of patients in the study while enhancing subsequent enrolment.9
Objectivity
VCTs, with their digitalised tools, sensors and electronic apps, will allow a more objective method of measuring results, allowing a better understanding of individualised drug response and adverse effects.9
Strengths and opportunities of VCTs in the context of dermatology – a match made in heaven?
Dermatology as a speciality relies primarily on visual diagnosis, which is supplemented by other diagnostic procedures. Correctly diagnosing from photographs adds credibility to a dermatologist’s repertoire, even though one would like it as a part of a quiz session rather than managing patients. VCTs would seem especially suited for a speciality like dermatology, extrapolating the success and suitability of telemedicine to dermatology. The increasing evidence that teledermatology shows good diagnostic concordance with face-to-face consultations, owing to better resolution photographs and the generally non-life threatening/emergency nature of skin conditions, further the case of VCTs in dermatology.
Threats and weaknesses of VCT in the context of dermatology
The regulatory framework for VCT approval for drug development is still nascent, and local/regional regulations must be factored in and tailor-made for each trial. Secondly, VCT is not technologically advanced enough for conducting phase I trials or in risky life-threatening conditions.5
Image-based diagnosis is often the cornerstone of dermatology VCTs, and all the limitations applicable to teledermatology play out here, too. Image quality, standardisation and consistency of pre-post images are key concerns. Ensuring image quality will require incorporating standardised imaging equipment and training to use the same (and the use of trained technicians), which could balance out some of the cost benefits otherwise associated with VCTs.
An obvious pitfall not necessarily related to dermatology is that therapeutic results cannot be accurately assessed on a virtual platform, as the thought process of the end user – the patient need not be the same as that of the initiator – the dermatologist.5
The older generation may not be well versed in modern technology, may be opposed to the absence of human interaction in the recruitment and monitoring process, and there is a risk of weakening the physician-patient relationship. VCT might not work if relevant laboratory investigations, physical examinations, etc., are not properly done by trained health staff during home visits or at a local designated centre.
The centre coordinating the VCT should have a robust information technology platform for smooth and competent operations. Internet connectivity is still a problem in rural and less connected areas.
Protecting patient privacy stored on servers and transferring sensitive health data online are problematic. Ethical aspects of deciding treatment outcomes based on remote evaluation are another concern.
In their early phases of development, wearable biometric devices have to be widely accepted by regulatory bodies after clinical validation. The collected data from electronic devices and wearable sensors should be accurate, reproducible and reliable. The cost of wearable devices also needs to be factored in.
VCTs require the shipping of drugs to multiple coordinating sites and, at times, directly to patient homes. The drug stability and appropriate storage facilities in the patient’s home, measures to prevent unauthorised access and communication between the drug source, storage and patient homes to provide timely refills to avoid study interruptions are essential.7
Future of VCTs and opportunities
Integrating specialities for VCTs (for example, radiologists, pathologists and dermatologists) is an exciting new avenue.
Certain organisations like Decentralised Trials and Research Alliance (DTRA) were launched to enable collaboration of stakeholders like pharmaceutical and biotechnology companies, regulatory authorities like the US-FDA, patient advocacy organisations, clinical research organisations, technology companies, specialised service providers, investigator site networks, consulting organisations, etc., to fast-track implementation of patient-focused VCTs within healthcare.9
Artificial intelligence (AI) in the form of blockchain, virtual or augmented reality, digital assistants, voice recognition, and digital biomarkers as primary and secondary endpoints have vast implications. AI tools to analyse and interpret unstructured data and dedicated apps that can further simplify the whole process will vastly advance the methodology and subsequently simplify the process, giving a huge boost to dermatology research.
Big data, a new method for complex large data, allows investigators to maximise the potential of existing data, offering a resourceful framework for further research and aid in CTs. Its use in dermatology can help improve risk prediction models, screening and surveillance of rare diseases, optimise and design tailor-made management of various disorders, and even offer clinicians a decision support system regarding the need for biopsy.10
The biggest opportunity of VCTs lies in the fact that while the enthusiasm, cost-effectiveness and efficiency of the drug development process are declining considerably, the understanding of disease aetiology and pathogenesis, the number of therapeutic targets and the need for novel medications are swiftly increasing. Hence, CT planning necessitates moving towards a more sustainable model.3 Combining VCTs/Big Data with artificial intelligence will probably pave the way for large multi-centric trials, which can significantly improve the quantity and quality of data.
Even though clearer definitions of VCTs and limitations need to be addressed and reviewed for better and large-scale acceptability, with the growing importance and acceptance of telemedicine and cutting-edge technology, it is becoming apparent that patient-centric VCTs will eventually help us move away from conventional CTs.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
References
- Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp Clin Trials Commun. 2018;11:156-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Translational research: 4 ways to fix the clinical trial. Nature. 2011;28:477:526-8.
- [CrossRef] [PubMed] [Google Scholar]
- Novel methods and technologies for 21st-century clinical trials. JAMA Neurology. 2015;72:582.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- IFPMA-Facts-And-Figures-2021. Available from: https://www.ifpma.org/wp-content/uploads/2021/04/IFPMA-Facts-And-Figures-2021.pdf [accessed on 2023 Dec 12]
- Virtual clinical trials: Perspectives in dermatology. Dermatology. 2020;236:375-82.
- [CrossRef] [PubMed] [Google Scholar]
- National academies of sciences, engineering, and medicine; Health and medicine division; Board on health sciences policy; Forum on drug discovery, development, and translation. virtual clinical trials: Challenges and opportunities: Proceedings of a workshop. In: Shore C, Khandekar E, Alper J, eds. PubMed. Washington (DC): National Academies Press (US); 2019. Available from: https://pubmed.ncbi.nlm.nih.gov/31334937/. [accessed on 2023 Dec 12]
- [Google Scholar]
- Web-based trial to evaluate the efficacy and safety of tolterodine ER 4 mg in participants with overactive bladder: REMOTE trial. Contemporary Clinical Trials. 2014;38:190-7.
- [CrossRef] [PubMed] [Google Scholar]
- Decentralized clinical trials (DCTs): A few ethical considerations. Front Public Health. 2022;10:1081150.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Decentralized clinical trials. JACC: Basic to Translational Science. 2021;6:384-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Research techniques made simple: An introduction to use and analysis of big data in dermatology. Journal of Investigative Dermatology.. 2017;137:e153-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





