Translate this page into:
Walking a day in a pachyonychia congenita patient’s shoes: Impact on plantar pain and activity levels measured with wristband activity trackers
Corresponding author: Dr. Shari R. Lipner, Department of Dermatology, Weill Cornell Medical College, New York, United States of America. shl9032@med.cornell.edu
-
Received: ,
Accepted: ,
How to cite this article: Lipner SR, Falotico JM, Matushansky JT, Evans H, Schwartz J, Hansen CD. Walking a day in a pachyonychia congenita patient’s shoes: Impact on plantar pain and activity levels measured with wristband activity trackers. Indian J Dermatol Venereol Leprol 2023;89:850-3.
Abstract
Background
Plantar keratoderma is a common finding in pachyonychia congenita, significantly impairing ambulation and quality of life. Due to the variation of pain reporting in pachyonychia congenita clinical studies, it is difficult to evaluate the efficacy of treatment outcomes for painful plantar keratodermas.
Objectives
To objectively analyse associations between plantar pain and activity levels in pachyonychia congenita patients using a wristband tracker.
Methods
Pachyonychia congenita patients and matched normal controls wore wristband activity trackers and completed a daily digital survey to record their highest and total pain scores (0–10 scale) each day for 28 consecutive days during four different seasons.
Results
Twenty four participants (12 pachyonychia congenita patients and 12 matched normal controls) completed the study. Pachyonychia congenita patients walked 1801.30 fewer steps/day (95% CI, −3666.4, 64.1) than normal controls (P = 0.072) and had greater average total [5.26; SD, 2.10] and highest (6.92; SD, 2.35) daily pain than normal controls [0.11 (SD, 0.47), 0.30 (SD, 0.22), respectively] (P < 0.001, both). On average, for each one unit increase in daily highest pain level, pachyonychia congenita activity decreased 71.54 steps/day (SE, 38.90, P = 0.066).
Limitation
The study had a small number of participants, limiting statistical power. Only pachyonychia congenita patients, ages 18 years or older, with keratin 6a, keratin 16, and keratin 17 mutations were included, limiting generalizability.
Conclusion
Pachyonychia congenita patients were less active with significantly higher pain than normal controls. There was an inverse correlation between pain and activity. Our findings suggest that wristband tracker technology may be used to evaluate treatment efficacy in future trials on severe plantar pain; therapeutic interventions that decrease plantar pain should correlate with significant increases in activity using wristband trackers.
Keywords
Activity level
pachyonychia congenita
plantar keratoderma
plantar pain
wristband tracker
Plain Language Summary
Pachyonychia congenita is a rare genetic disease affecting the skin and nails. Many patients develop thickened skin on the soles of their feet, causing difficulty in walking and performing daily activities. It is difficult to determine if studied treatments reduce plantar pain because reporting is subjective. In this study, we looked for relationships between foot pain and activity levels using wristband trackers. Pachyonychia congenita patients and normal controls wore wristband trackers and recorded their highest and total pain scores each day. We found that pachyonychia congenita patients walked fewer steps than normal controls and had significantly greater highest and total daily pain, on average. We also found that as the pain increased, the number of steps walked decreased. These findings suggest that the wristband tracker can be used to study relationships between pain and activity. This technology may also be used in future studies evaluating therapies for foot pain.
Introduction
Pachyonychia congenita (PC) is a rare autosomal dominant disorder caused by mutations in keratin genes KRT6A, KRT6B, KRT6C, KRT16 and KRT17, which encode keratins 6a, 6b, 6c, 16 and 17, respectively.1,2 Painful plantar keratoderma is the most prominent symptom in patients with PC and significantly impacts daily function and quality of life.3,4 Currently, there is no effective topical or systemic intervention for the plantar keratoderma associated with PC. The current standard of care consists of routine removal of calluses followed by treatment with moisturisers.4,5 There is a need for the development of new and effective therapies for the management of plantar keratoderma, but the lack of standardized pain reporting in pachyonychia congenita clinical studies makes meaningful interpretation of treatment outcomes challenging. Given that plantar keratoderma-associated pain significantly impacts ambulation and quality of life,6 we aimed to objectively analyze associations between plantar pain and activity levels using a wristband tracker.
Materials and Methods
Institutional Review Board approval was obtained from the Western Institutional Review Board (study number 1161360). Pachyonychia congenita (PC) patients, ages 18 years or older, with KRT6A, KRT16, and KRT17 mutations, enrolled in the International Pachyonychia Congenita Research Registry with reported plantar keratoderma in previous International Pachyonychia Congenita Research Registry questionnaires, were recruited and paired with normal controls, matched for age (within 5 years), sex, and geographic location (within 50 miles). All participants (PC patients and normal controls) signed a written informed consent form agreeing to participate in the trial. Exclusion criteria were non-English speakers, patients with secondary skin infections, diabetes, or other non-pachyonychia congenita-related diseases.
Participants wore wristband activity trackers (Withings, Activité Pop Tracker v2.0, France) and completed a digital survey reporting their daily highest and total pain scores on a 0–10 scale (0 = no pain, 10 = worst pain) each day for 28 consecutive days during four different seasons: spring (March 2016), summer (July 2016), fall (October 2016), winter (January 2017) (112 days total). Total and highest daily pain were patient-reported outcomes on the digital survey, which could be saved on a mobile device to operate like a smartphone application for easier daily access. Participants were instructed to wear the tracker on their wrist for 24 hours a day during each study phase, to continue their normal routines and activity, and to open the Withings Health Mate smartphone application (free access, available on the application store for both Android and iOS systems) once each day, where data from the activity tracker was downloaded and transmitted to the study team. Participants did not receive any other plantar keratoderma-related treatments (i.e., botulinum toxin injections, keratolytics, oral retinoids, etc.) during the duration of the study.
Univariate linear mixed models were used to evaluate between-group differences in steps as well as pain scores over repeated assessments (i.e., daily measurements) and to account for potential missing data at one or more of the evaluation time points. All P values were two-sided, with statistical significance evaluated at the 0.05 α level. 95% confidence intervals for all parameters were calculated to assess the precision of the obtained estimates. Analyses were performed in R Version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 24 participants (12 pachyonychia congenita patients, 12 normal controls) completed the study. Average age (47.2 ± 11.1 years), weight (73.70 ± 14.31 kilograms), and height (1.66 ± 0.07 meters) of PC patients did not differ from normal controls (47.9 ± 10.2 years, 75.48 ± 16.74 kilograms, 1.65 ± 0.06 meters, respectively) (P > 0.05, all) [Table 1]. Overall, mean steps/day for PC patients were (4068.9 ± 2114.3) and for normal controls were (5931.0 ± 4152.5). PC patients walked 1801.30 fewer steps/day (95% confidence interval, −3666.4, 64.1) than normal controls (P = 0.072). Average total pain (5.26 ± 2.10) and highest daily pain (6.92 ± 2.35) for PC patients were greater than normal controls (0.11 ± 0.47, 0.30 ± 0.22, respectively) (P < 0.001, both) [Figure 1]. Highest pain in PC patients did not differ seasonally (P > 0.05, all). Total pain was 0.199 points lower in spring vs. winter (standard error, 0.10; P = 0.037). On average, for each unit increase in daily highest pain level, activity of pachyonychia congenita patients decreased by 71.54 steps/day (standard error, 38.90; P = 0.066) [Table 2].
| Participant | Gene | Mutation | Sex | Age, years (SD) | State/province | Weight, kilograms (SD) | Height, meters (SD) |
|---|---|---|---|---|---|---|---|
| PC1 | K16 | K15X | F | 40 | New York | 77.10 | 1.63 |
| PC2 | K16 | M121R | M | 43 | Ontario | 84.23 | 1.73 |
| PC3 | K16 | N125S | F | 37 | Nevada | 58.06 | 1.63 |
| PC4 | K16 | R127C | F | 58 | Minnesota | 96.12 | 1.70 |
| PC5 | K16 | R127C | F | 58 | Colorado | 69.49 | 1.63 |
| PC6 | K16 | R127C | F | 50 | Colorado | 58.06 | 1.63 |
| PC7 | K17 | E111A | F | 50 | South Carolina | 85.82 | 1.63 |
| PC8 | K6a | D432_E470dup | F | 50 | Pennsylvania | 43.99 | 1.60 |
| PC9 | K6a | E472K | M | 20 | West Virginia | 81.60 | 1.83 |
| PC10 | K6a | N171K | F | 47 | Utah | 77.50 | 1.63 |
| PC11 | K6a | V181_Q186del | F | 55 | Oregon | 77.11 | 1.60 |
| PC12 | K6a | V181_Q186del | F | 58 | California | 75.30 | 1.65 |
| PC Average | N/A | N/A | N/A | 47.2 (11.1) | N/A | 73.70 (14.31) | 1.66 (0.07) |
| NC1 | N/A | N/A | F | 44 | Nevada | 90.72 | 1.63 |
| NC2 | N/A | N/A | M | 42 | Ontario | 104.30 | 1.70 |
| NC3 | N/A | N/A | F | 39 | Wyoming | 54.88 | 1.63 |
| NC4 | N/A | N/A | F | 56 | Minnesota | 77.10 | 1.63 |
| NC5 | N/A | N/A | F | 57 | Colorado | 56.60 | 1.70 |
| NC6 | N/A | N/A | F | 47 | Colorado | 79.38 | 1.70 |
| NC7 | N/A | N/A | F | 55 | South Carolina | 55.79 | 1.63 |
| NC8 | N/A | N/A | F | 52 | Pennsylvania | 90.70 | 1.68 |
| NC9 | N/A | N/A | M | 23 | West Virginia | 63.59 | 1.70 |
| NC10 | N/A | N/A | F | 47 | Utah | 80.65 | 1.75 |
| NC11 | N/A | N/A | F | 53 | Oregon | 90.72 | 1.52 |
| NC12 | N/A | N/A | F | 60 | California | 61.37 | 1.57 |
| NC Average | N/A | N/A | N/A | 47.9 (10.2) | N/A | 75.48 (16.74) | 1.65 (0.06) |
F: female, M: male, N/A: not applicable, NC: normal control, PC: pachyonychia congenita, SD: standard deviation
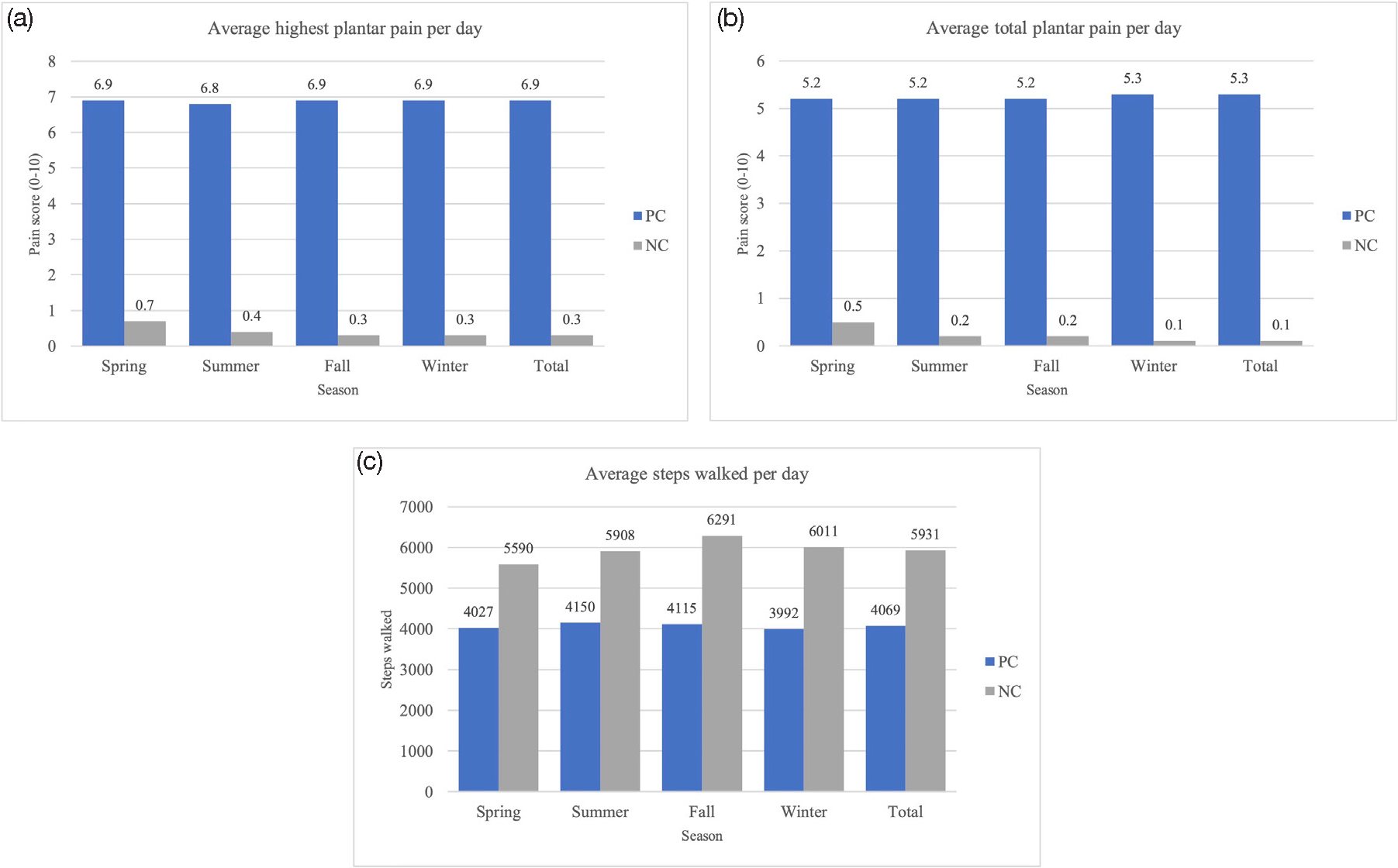
- Average highest pain scores (a), total pain scores (b), and steps walked (c) per day for PC and NC participants across four seasons. NC: normal control, PC: pachyonychia congenita. Daily activity (steps walked) and pain levels (total and highest) were compared seasonally, with winter used as the reference season.
| Steps (activity) | Highest pain | Total pain | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P- value | Estimate | SE | P- value | Estimate | SE | P- value | |
| PC vs NC | −1801.30 | 954.48 | 0.072 | 6.30 | 0.55 | <0.001 | 4.80 | 0.61 | <0.001 |
| Season* (PC only) | |||||||||
| Season 1 vs Season 4 | 41.85 | 119.45 | 0.726 | −0.14 | 0.10 | 0.168 | −0.20 | 0.10 | 0.037 |
| Season 2 vs Season 4 | 200.48 | 124.84 | 0.109 | −0.12 | 0.11 | 0.272 | −0.06 | 0.10 | 0.513 |
| Season 3 vs Season 4 | 168.66 | 123.07 | 0.171 | −0.06 | 0.11 | 0.566 | −0.05 | 0.10 | 0.617 |
| Keratin 6a vs Keratin 16 | −967.49 | 732.94 | 0.219 | --- | --- | --- | --- | --- | --- |
| Steps (activity) (PC only) | --- | --- | --- | −71.54 | 38.90 | 0.066 | −70.09 | 44.49 | 0.115 |
NC: normal control, PC: pachyonychia congenita, SE: standard error. *Season 1: spring, season 2: summer, season 3: fall, season 4: winter
Discussion
Our study is the first to objectively measure activity levels in pachyonychia congenita (PC) patients using standardized wristband trackers and to correlate daily activity with pain levels. In a survey of 254 genetically confirmed PC patients, 95% (n = 241/254) reported plantar keratoderma and 89% (n = 225/254) reported plantar pain.7 Therefore, we theorized that plantar pain, which is a defining feature of PC patients, would impact activity. We found a trend towards PC patients being less active, with significantly higher pain levels than matched normal controls.
There are no standardized treatment regimens for PC-related plantar pain. Current therapies provide only temporary and partial pain control.8 In a survey of 113 PC patients with plantar keratoderma, 82.4% of conventional therapies were rated as marginally or poorly effective.5 Since it is challenging to execute controlled clinical trials on patients with painful keratodermas such as PC, most treatment recommendations are anecdotal or based on small case reports or case series. Currently, there is a phase-Ib clinical study evaluating the efficacy and safety of topical 1% sirolimus (a mammalian target of rapamycin inhibitor) cream in treating plantar keratoderma in adult PC patients (https://clinicaltrials.gov/ct2/show/NCT02152007). In our study, we showed an inverse correlation between pain and activity. Therefore, in future clinical trials evaluating the treatment of plantar keratoderma-associated pain, therapeutic interventions that decrease plantar pain should correlate with significant increases in activity as measured on the wristband tracker.
Based on case reports, pain in some PC patients worsens during the summer.9 It is theorized that excessive sweating results in subepidermal blisters, with pressure stimulating sensory nerve fibres and causing pain.8 However, we did not observe seasonal differences in activity levels and total pain was actually lower in warmer months.
Due to the rarity of PC, it is difficult to recruit a large number of patients for clinical trials. Therefore, our study had small numbers, limiting statistical power and potentially contributing to false negative P values (β error). Furthermore, due to the small sample size, we could not match participants by occupation or lifestyle (i.e., sedentary), which may contribute to differences in steps walked per day. Outlier data (i.e., participants who were more active than others) was included in the analysis. We only included PC patients with KRT6a, KRT16, and KRT17, mutations who were 18 years old or older, limiting generalizability. The accuracy of activity trackers can vary based on the tracker, software, and type of activity, which may also affect generalizability. One normal control participant dropped out of the study after the first session.
Conclusion
In this study, we used a wristband tracker to document pachyonychia congenita patients being less active and having significantly higher pain than normal controls. This pilot study shows proof of concept that this wristband technology can assess pain and activity associations. Future studies are needed to corroborate these findings in larger cohorts of pachyonychia congenita patients and/or other patients who suffer from severe plantar pain. Our current findings suggest that the wristband tracker may be used in conjunction with traditional methods of pain reporting (i.e., visual analogue scale scores) to evaluate treatment efficacy in future trials on severe plantar pain, with treatments successful in decreasing pain demonstrating increased activity using wristband trackers.
Declaration of patient consent
Patient’s consent is not required as the patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflict of interest
Ms Falotico, Mr Matushansky, Ms Evans, and Ms Schwartz have no conflicts of interest to disclose. Dr Lipner has served as a consultant for Ortho Dermatologics, BelleTorus Corporation, and Hoth Therapeutics. Dr Hansen serves on the non-profit Pachyonychia Congenita Board and is the principal investigator for the International Pachyonychia Congenita Research Registry.
References
- The phenotypic and molecular genetic features of pachyonychia congenita. J Invest Dermatol. 2011;131:1015-7.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of clinically useful predictive genetic variants in pachyonychia congenita. Clin Exp Dermatol. 2021;46:867-73.
- [CrossRef] [PubMed] [Google Scholar]
- Distinctions in the management, patient impact, and clinical profiles of pachyonychia congenita subtypes. Skin Appendage Disord. 2021;7:163-71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pathophysiology of pachyonychia congenita‐associated palmoplantar keratoderma: New insights into skin epithelial homeostasis and avenues for treatment. Br J Dermatol. 2020;182:564-73.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Best treatment practices for pachyonychia congenita. J Eur Acad Dermatol Venereol. 2014;28:279-85.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and pathological features of pachyonychia congenita. J Investig Dermatol Symp Proc. 2005;10:3-17.
- [CrossRef] [PubMed] [Google Scholar]
- A review of the clinical phenotype of 254 patients with genetically confirmed pachyonychia congenita. J Am Acad Dermatol. 2012;67:680-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pain mechanisms in hereditary palmoplantar keratodermas. Br J Dermatol. 2020;182:543-51.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of pachyonychia congenita with plantar injections of botulinum toxin. Br J Dermatol. 2006;154:763-5.
- [CrossRef] [PubMed] [Google Scholar]







