Translate this page into:
Weekly azathioprine pulse versus daily azathioprine in the treatment of Parthenium dermatitis: A non-inferiority randomized controlled study
2 Department of Biostatistics, All India Institute of Medical Sciences, New Delhi - 110 029, India
Correspondence Address:
Kaushal K Verma
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi - 110 029
India
| How to cite this article: Verma KK, Sethuraman G, Kalavani M. Weekly azathioprine pulse versus daily azathioprine in the treatment of Parthenium dermatitis: A non-inferiority randomized controlled study. Indian J Dermatol Venereol Leprol 2015;81:251-256 |
Abstract
Background: Azathioprine in daily doses has been shown to be effective and safe in the treatment of Parthenium dermatitis. Weekly pulses of azathioprine (WAP) are also effective, but there are no reports comparing the effectiveness and safety of these two regimens in this condition. Aims: To study the efficacy and safety of WAP and daily azathioprine in Parthenium dermatitis. Methods: Sixty patients with Parthenium dermatitis were randomly assigned to treatment with azathioprine 300 mg weekly pulse or azathioprine 100 mg daily for 6 months. Patients were evaluated every month to assess the response to treatment and side effects. Results: The study included 32 patients in the weekly azathioprine group and 28 in the daily azathioprine group, of whom 25 and 22 patients respectively completed the study. Twenty-three (92%) patients on WAP and 21 (96%) on daily azathioprine had a good or excellent response. The mean pretreatment clinical severity score decreased from 26.4 ± 14.5 to 4.7 ± 5.1 in the WAP group, and from 36.1 ± 18.1 to 5.7 ± 6.0 in the daily azathioprine group, which was statistically significant and comparable (P = 0.366). Patients on WAP had a higher incidence of adverse effects (P = 0.02). Limitations: The study had a small sample size and the amount of clobetasol propionate used in each patient was not determined, though it may not have affected the study outcome due to its comparable use in both groups. Conclusions: Azathioprine 300 mg weekly pulse and 100 mg daily dose are equally effective and safe in the treatment of Parthenium dermatitis.INTRODUCTION
Air-borne contact dermatitis to Parthenium hysterophorus (Parthenium dermatitis), is the most common type of airborne contact dermatitis in India, [1] and is a cause of significant dermatological morbidity in our country. [2] Corticosteroids have been the mainstay of treatment for this condition [2] but owing to its chronic nature, the long term use of corticosteroids often causes severe and sometimes, irreversible side effects. [3],[4] Azathioprine, an immunosuppressive drug, which acts by inhibiting the activated T lymphocytes, has emerged as an effective and safe alternative. [5] In earlier studies, we demonstrated that azathioprine 100 mg daily was effective, [6],[7],[8] safe for long-term use, and could induce lasting remissions in Parthenium dermatitis. [9] In a small open study, we also demonstrated that 300 mg weekly pulse doses of azathioprine was not only effective and safe in the treatment of Parthenium dermatitis, but reduced the cost of treatment by 60% and encouraged compliance (since only four doses of the drug were taken in a month). [10] We designed this non-inferiority trial to compare the effectiveness and safety of weekly pulse with daily doses of azathioprine in patients with air-borne contact dermatitis to Parthenium.
METHODS
Patients with a clinical diagnosis of Parthenium induced air-borne contact dermatitis attending the dermatology outpatient department of the All India Institute of Medical Sciences, New Delhi, India between September 2006 and August 2010 were selected for the study. The study was approved by the Institutional Ethics Committee. Patients below the age of 15 years, pregnant and lactating women, or those with abnormal baseline hematological, liver, or renal function tests were excluded. Written informed consent was taken from all patients. A random allocation sequence was generated using a random number table. Identical sealed brown paper packets containing either 60 or 24 tablets of azathioprine (50 mg) were numbered according to the allocation sequence. Patients were randomly assigned to treatment with azathioprine weekly pulse with 6 tablets every week (weekly azathioprine group) or azathioprine 2 tablets daily (daily azathioprine group), for 6 months in a blinded manner. Antihistamines (cetrizine hydrochloride, 10 mg) once daily orally and clobetasol propionate (0.05% w/w) cream topically were given to all the patients for symptomatic relief initially.
No other drugs (including medications of alternative systems) were permitted. All patients were counseled to use protective clothing and frequently wash the exposed areas with soap and water.
A detailed clinical evaluation was undertaken in each patient. Disease severity was assessed by the clinical severity score (CSS) based on (a) itching, (b) erythema, (c) morphology of skin lesions, and (d) area of involvement. Itching and erythema were graded on a scale of 0 to 3 (0: Nil; 1: Mild; 2: Moderate; and 3: Severe) and morphology was graded on a scale of 0-5 (0: No lesions; 1: Papules; 2: Plaques; 3: Lichenified papules; 4: Lichenified plaques; 5: Exudative lesion). The area of involvement, was graded using a method described by us earlier. [10] Patch testing with a standardized aqueous extract of the plant antigen [11] was performed to confirm the diagnosis. Tenfold aqueous dilutions of the standard extract from 1:10 to 1:100,000 dilutions were used to determine the titer of contact hypersensitivity (TCH). [12] The maximum dilution, which produced a definite dermatitic reaction at the patch test site, was taken as the titer of contact hypersensitivity in that patient. Laboratory investigations including hemoglobin, total blood count, differential count, platelets, serum bilirubin, alkaline phosphatase, transaminases, creatinine, electrolytes, fasting blood sugar, blood urea, urine routine and microscopy, stool examination for occult blood, chest X-ray, and electrocardiogram (ECG) were carried out at the start of therapy and at each subsequent visit. Blood pressure and weight were recorded, and clinical photographs of each patient were taken. Thiopurine methyltransferase (TPMT) enzyme activity was not estimated owing to lack of facilities.
Patients were evaluated monthly to assess the response and adverse effects of the therapy. At each visit the clinical severity score, flattening and healing of the lesions, occurrence of new lesions and overall improvement were assessed. After 6 months, treatment was stopped and the final response to treatment was assessed in both groups. The response was considered to be excellent if the overall improvement was 75-100%; good, if it was 50-75%; and poor, if it was below 50%. The patients in both the groups were followed-up every month for 6 months after stopping the treatment to determine any relapse of the disease. The disease was considered to have relapsed if the clinical severity score increased to more than 50% of the pretreatment level.
Statistical analysis
Statistical analysis was carried with a data analysis and statistical software (STATA 9.0; College station, Texas, USA). Baseline characteristics were presented as number (%) or medium (range) as appropriate and compared between the two groups using Student′s ′t′ test/Wilcoxon ranksum test or Chi-square test as appropriate. The primary outcome was analyzed by both intention-to-treat (ITT) and per-protocol method. Missing values were replaced by the worst case scenario in the ITT method. Non-inferiority was tested using confidence interval (one sided) approach. The results were presented as absolute difference (95% CI). The relative risk (95% CI) was also calculated.
RESULTS
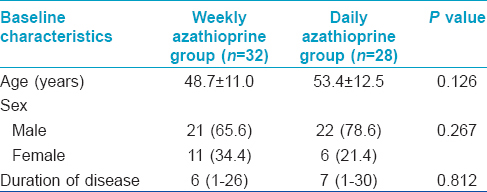
Of the 72 patients with Parthenium dermatitis who were screened, 60 (43 males and 17 females; mean age 50.9 years (range 30 and 72 years); mean disease duration 7.9 yrs (range 1-30 years)) were included in the study. Forty seven patients (33 males and 14 females) completed the study. There were 25 patients in the WAP group and 22 in the daily azathioprine group [Figure - 1]. The baseline demographic characteristics of the patients in the two groups were comparable [Table - 1]. Five patients (3 in Group A and 2 in Group B) were lost to follow-up; of these, one patient consulted a local doctor for treatment, but reasons for not following up was not known for the rest. Six patients (3 in each group) were withdrawn from the study. In 5 of these 6 patients severe azathioprine induced nausea/vomiting, diarrhea and abdominal pain (3 and 2 patients each in WAP and daily azathioprine groups respectively) lead to discontinuation of treatment and 1 patient in Group B was diagnosed to have pulmonary tuberculosis. Two patients were excluded from the final analysis, 1 patient in Group B failed to follow-up at the 5 th month, while the other from Group A discontinued therapy after developing chickenpox.
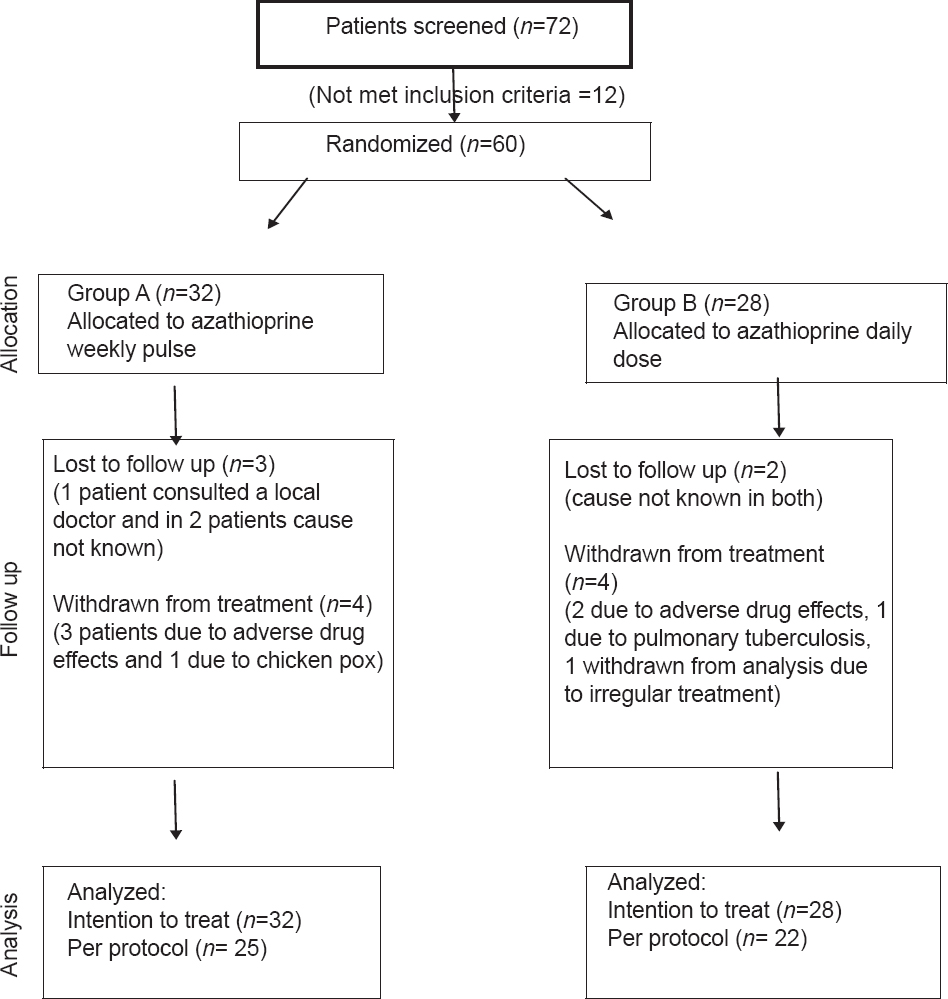 |
| Figure 1: Study flow chart showing randomization, treatment assignment, follow-up and analysis |
Response to treatment
A total of 25 patients in Group A (median duration of disease 6 years; range 1-26 years) and 22 patients in Group B (median duration of disease 7 years; range 1-30 years) completed the study.
ITT and per-protocol analysis were performed to compare the treatment outcomes. The primary endpoint was ≥50% change in clinical severity score from baseline to 6 months (excellent to good response) with a non-inferiority margin (∆) of −15%. The rate of excellent to good responses was 81.3% and 89.3% in Group A and Group B, respectively (a difference of −8.0% (−22.0% to 6.8%), which is not statistically significant).
Twenty-three (92%) patients in Group A and 21 (95%) patients in Group B had a good or excellent response, while 2 (8%) patients in Group A and 1 (5%) in Group B had a poor response as per-protocol analysis. Twenty-six (81%) patients in Group A and 25 (89%) patients in Group B had ≥50% reduction in their clinical severity score from baseline on ITT analysis indicating excellent to good response, while 6 (19%) patients in Group A and 3 (11%) in Group B had poor response [Table - 2]. The mean pretreatment clinical severity score decreased from 26.4 ± 14.5 to 4.7 ± 5.1 in the weekly azathioprine group, while it decreased from 36.1 ± 18.1 to 5.7 ± 6.0 in daily azathioprine group [Figure - 2]. The good response (CSS≥50) with weekly azathioprine was no worse than 3.5% (per protocol) as compared to daily azathioprine (95.5% in daily azathioprine vs 92.0% in weekly azathioprine) with the lower 95% confidence interval being -14.8% which is greater than predefined non-inferiority margin (∆=-15%).
 |
| Figure 2: Clinical severity score over a period of time between two groups |

Titer of contact hypersensitivity
The changes in titer of contact hypersensitivity were comparable in both the groups. It decreased in 11 and 14 patients in Group A and Group B respectively, and became negative in 1 patient in each of the groups. The titer increased in 8 and 5 patients respectively in Groups A and B.
The titer of contact hypersensitivity was not repeated on completion of treatment in two patients in the weekly azathioprine group and in one patient in the daily azathioprine group.
Adverse effects
Nausea and vomiting were the most frequent adverse effects in the weekly azathioprine group (statistically significant, Fisher exact ≤0.05); these were managed conservatively without stopping treatment. Other adverse effects were statistically not significant in either group [Table - 3]. Transient changes in hematological and biochemical parameters (statistically not significant; P ≥ 0.05) became normal during the follow-up while continuing therapy in both the groups.

Relapse
Sixteen patients in Group A and 15 patients in Group B completed 6 months′ post-treatment follow-up; of these, 4 (25%) patients and 2 (13%) patients respectively had a relapse of the disease. Relapses occurred after 4 months in 1 patient and 6 months in 3 patients in Group A; in Group B however, 1 patient relapsed after 1 month and another at 2 months. The relapse rate between the two groups was not statistically significant (P > 0.05).
DISCUSSION
Parthenium dermatitis is a common chronic allergic disease which affects a large number of patients attending the contact dermatitis clinics in our country. The disease is more severe during summer and monsoon due to the presence of large amount of air-borne allergens, sweating and increased skin exposure to environment due to light clothing etc. Corticosteroids are the first-line drugs used in the treatment of Parthenium dermatitis. However owing to its chronicity, these patients require prolonged treatment with corticosteroids which often leads to corticosteroids induced side effects, some of which could be severe and irreversible. [3],[4]
Azathioprine, a 6-mercaptopurine derivative, is a strong immunosuppressive agent which acts by inhibiting activated T-lymphocytes and also has a potent anti-inflammatory effect. It has been demonstrated to be very effective in the treatment of Parthenium dermatitis [7],[8] due to its profound effect on T-lymphocytes, [5] the cells mainly responsible for causing dermatitis. The safety and toxicity of azathioprine have been well studied and the drug has been found to be safe even on long term use without significant side effects. [9]
In this double-blind non-inferiority randomized study, weekly pulse doses and daily doses of azathioprine were compared in the treatment of Parthenium dermatitis. There was excellent to good response in 92% (23/25) patients in weekly azathioprine pulse group and 95% (21/22) patients in daily azathioprine group on per protocol analysis. However, 81% (26/32) patients in weekly azathioprine group and 89% (25/28) patients in daily azathioprine group had ≥50% reduction in their clinical severity score from baseline on ITT analysis indicating an excellent to good response [Table - 2]. The response to treatment in both the groups was highly significant and comparable (P = 0.366).
The mean pre-treatment clinical severity score decreased from 26.4 ± 14.5 to 4.7 ± 5.1 in weekly azathioprine group while it decreased from 36.1 ± 18.1 to 5.7 ± 6.0 in daily azathioprine group ([Figure - 2]). Although the response with the WAP regimen was 3.5% less (pre protocol) and 8% less (as per ITT) than with weekly azathioprine pulse, this was within the confidence limits (15%).
In a study by Verma et al., 95% patients of Parthenium dermatitis had excellent response with daily doses of azathioprine and there was a decrease in clinical severity score from 64.5 ± 16.3 to 4.3 ± 5.57 (P = 0.01). [8] Similarly in another study, azathioprine weekly pulse dose showed an excellent response in 58% (7/12) patients and good response in 42% (5/12) patients with a reduction in clinical severity score from 40.40 ± 7.95 to 10.9 ± 8.43 (P = 0.002) which was statistically highly significant and the therapy was well tolerated without any significant side effects. [10] There were no significant changes in the laboratory parameters in any of these patients. [10] In the present study the response to treatment with two regimens i.e. azathioprine weekly pulse and azathioprine daily dose are comparable with the earlier studies. [8],[10] Gastrointestinal side effects were, however, more frequently observed in azathioprine weekly pulse group (P = 0.02) [Table - 3] which were managed conservatively without stopping therapy in any patient. Also there were no significant changes in titer of contact hypersensitivity value in either group which is consistent with our earlier observations, showing no correlation between the titer of contact hypersensitivity and severity of disease and/or response to treatment. [13] Therefore, determination of titer of contact hypersensitivity is probably not helpful to assess the treatment response and is not recommended. Transient changes in hematological and biochemical parameters were seen in some patients in both the groups which were comparable and statistically not significant (P ≥ 0.05). The changes returned to normal during the follow-up while continuing therapy.
The thiopurine methyltransferase (TPMT) estimation prior to starting these patients on azathioprine was not done due to lack of laboratory facilities. Moreover it has been shown that the prospective estimation of TPMT enzyme activity does not predict azathioprine induced adverse effects. [14] Also the study by Benkov et al. does not recommend routine TPMT estimation in such patients. [15] A routine monitoring with haematological and biochemical tests is considered adequate.
In conclusion, the present study has demonstrated that azathioprine administered as 300 mg weekly pulse is as effective as azathioprine 100 mg daily in the treatment of Parthenium dermatitis. Additionally, the cost of the therapy is reduced by 60% with this regimen and compliance to treatment is expected to be better even on long-term use owing to the convenient once weekly dose. The only significant adverse effect was nausea/vomiting.
However, our study has the following limitations: (i) small sample size (ii) exact amount of topical clobetasol propionate used by each patient was not determined. The latter is unlikely to have affected the results since the mean pretreatment clinical severity score were similar in both groups and hence the patients in both the groups would be expected to use comparable amounts of the drug. Larger studies, however, are required to confirm our results.
ACKNOWLEDGMENT
The authors gratefully acknowledge the help of Prof. R. M .Pandey in statistical analysis of study data. The authors also thank Dr. Ansul Avijit and Dr. Pradeep Kumar who worked as Senior Research Fellows in the study.
| 1. |
Sharma SC, Kaur S. Contact dermatitis from composite plants. Indian J Dermatol Venereol Leprol 1990;56:27-30.
[Google Scholar]
|
| 2. |
Verma KK, Sirka CS, Ramam M. Contact dermatitis due to plants: Challenges and prospects. Indian Pract 2001;54:791-6.
[Google Scholar]
|
| 3. |
Gallant C, Kenny P. Oral glucocorticoids and their complications: A review. J Am Acad Dermatol 1968;14:161-7.
[Google Scholar]
|
| 4. |
Storrs FJ. Use and abuse of systemic corticosteroid therapy. J Am Acad Dermatol 1979;1:95-106.
[Google Scholar]
|
| 5. |
Thomas CW, Myhre GM, Tschumper R, Sreekumar R, Jelinek D, McKean DJ, et al. Selective inhibition of inflammatory gene expression in activated T lymphocytes: A mechanism of immune suppression by thiopurines. J Pharmacol Exp Ther 2005;312:537-5.
[Google Scholar]
|
| 6. |
Verma KK, Pasricha JS. Azathioprine as a corticosteroid-sparing agent in air borne contact dermatitis. Indian J Dermatol Venereol Leprol 1996;62:30-2.
[Google Scholar]
|
| 7. |
Verma KK, Manchanda Y, Pasricha JS. Azathioprine as a corticosteroid sparing agent for the treatment of dermatitis caused by the weed parthenium. Acta Dermatol Venereol 2000;80:31-2.
[Google Scholar]
|
| 8. |
Verma KK, Mahesh R, Srivastava P, Ramam M, Mukhopadhyaya AK. Azathioprine versus betamethasone for the treatment of parthenium dermatitis: A randomized controlled study. Indian J Dermatol Venereol Leprol 2008;74:453-7.
[Google Scholar]
|
| 9. |
Verma KK, Manchanda Y. Long-term safety and toxicity of azathioprine in patients with air-borne contact dermatitis. Indian J Dermatol Venereol Leprol 2000;67:75-7.
[Google Scholar]
|
| 10. |
Verma KK, Bansal A, Sethuraman G. Parthenium dermatitis treated with azathioprine weekly pulse doses: An open study. Indian J Dermatol Venereol Leprol 2006;72:24-7.
[Google Scholar]
|
| 11. |
Pasricha JS, Singh SN. Evaluation of antigen impregnated discs for patch tests. Indian J Dermatol Venereol Leprol 1982;48:327-9.
[Google Scholar]
|
| 12. |
Pasricha JS. Titre of contact hypersensitivity (TCH) as a means of determining the degree of contact hypersensitivity in contact dermatitis. Indian J Dermatol Venereol Leprol 1986;52:195-7.
[Google Scholar]
|
| 13. |
Verma KK, Manchanda Y, Dwivedi SN. Failure of titre of contact hypersensitivity to correlate with clinical severity and therapeutic response in contact dermatitis caused by Parthenium. Indian J Dermatol Venereol Leprol 2004;70:210-13.
[Google Scholar]
|
| 14. |
Sayani FA, Prosser C, Bailey RJ, Jacobs P, Fedorak RN. Thiopurine methyltransferase enzyme activity determination before treatment of inflammatory bowel disease with azathioprine: Effect on cost and adverse events. Can J Gastroenterol 2005;19:147-51.
[Google Scholar]
|
| 15. |
Benkov K, Lu Y, Patel A, Rahhal R, Russell G, Teitebaum J. Role of thiopurine metabolite testing and thiopurine methyltransferase determination in pediatric IBD. J Pediatr Gastroenterol Nutr, 2013;56:333-40.
[Google Scholar]
|
Fulltext Views
4,924
PDF downloads
2,987





